Group News & Updates
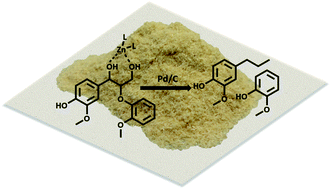
While current biorefinery processes use lignin only for its heat value, the conversion of lignin to high value chemicals is an area of increasing interest. Herein we present a detailed mechanistic study of the hydrodeoxygenation (HDO) of lignin by using a synergistic Pd/C and ZnII catalyst through use of both lignin model compounds and lignocellulosic biomass. Spectroscopic data coupled with the study of lignin model compounds suggest that ZnII activates and facilitates removal of the hydroxyl group at the Cγ position of the β-O-4 ether linkage. Activation is proposed to occur through formation of a six-membered ring complex of ZnII coordinated to the oxygen atoms at Cα and Cγ of the lignin model compound guaiacylglycerol-β-guaiacyl. Read More>>
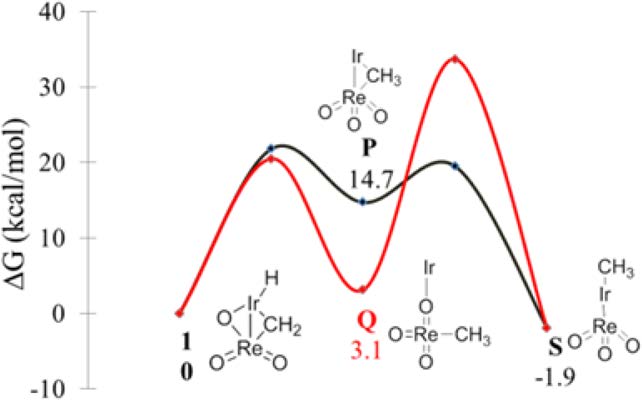
Investigation of the mechanism of conversion of the bimetallic
complex [PNP(H)Ir-μ(CH2)-μ(O)-Re(O)2][PF6] (1) to its structural isomer
[PNP(Me)(CH3CN)Ir-ReO3][PF6] (2) by detailed kinetics and DFT computational
studies is reported. The reaction proceeds by intramolecular rearrangement of 1 to
[PNP(Me)Ir-ReO3][PF6] (S) via a methyl-bridged [PNP(H)Ir-μ(CH3)-Re(O)3]-
[PF6] (P) intermediate followed by CH3CN coordination. The rate-determining step
is the transformation of 1 to P displaying ΔG⧧ of 21.8 kcal/mol. Experimental kinetic
results include zero-order dependence on acetonitrile, positive ΔS⧧, and deuteration
of the bridging methylidene group in the reaction of 1 with CD3OD. All of these
results support the proposed mechanism. Read More>>
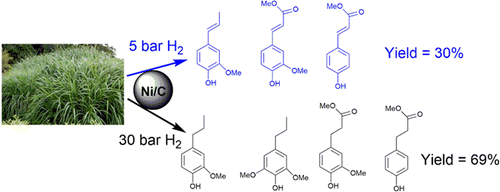
A two-step catalytic conversion of raw biomass into different high value chemicals (phenol products, levulinic acid and furfural) was established using earth abundant Ni/C and FeCl3 catalysts. Read More>>
|

