

Why do we want to know the structure for a coordination compound? The compound's structure (i.e., how the ligands are arranged around the metal atom) determines its physical and chemical properties. For example, a tetrahedral compound will behave differently than a square planar compound that contains the same metal atom and the same ligands.
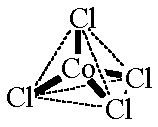
Consider the complex ion, [CoCl4]2-. The coordination number of [CoCl4]2- is equal to 4; thus, the structure of [CoCl4]2- might be:
| A Comparison of Tetrahedral and Square Planar Structures | ||
|---|---|---|
| example | sketch | model |
| [CoCl4]2- tetrahedral CN = 4 ligands attached at the corners of a tetrahedron |
 |
|
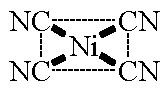
| [Ni(CN)4]2- square planar CN = 4 ligands attached at the corners of a square |
 |
Coordination numbers range from 1 to 12, with 2, 4 and 6 being the most common. Linear and octahedral are the most common structures for coordination numbers 2 and 6, respectively.
| A Comparison of Linear and Octahedral Structures | ||
|---|---|---|
| example | sketch | model |
| [Ag(NH3)2]+ linear CN = 2 ligands and metal atom connected in a straight line |
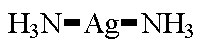 |
|
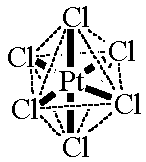
| [PtCl6]2- octahedral CN = 6 ligands attached at the corners of an octahedron |
 |
