Hazards Associated with Cryogens -- Pressure buildup
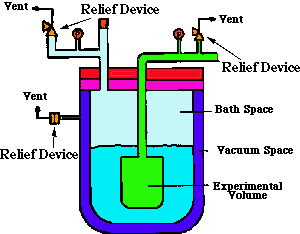 This is the most common cryogen hazard. Cryogens
boil as they sit in their storage vessels absorbing bits of
energy from the (much warmer) surroundings. The gas boiling out of
the liquid must either expand or the pressure will increase.
This is the most common cryogen hazard. Cryogens
boil as they sit in their storage vessels absorbing bits of
energy from the (much warmer) surroundings. The gas boiling out of
the liquid must either expand or the pressure will increase.
One volume of liquid nitrogen, for example, can quickly become 700
volumes of nitrogen gas. Or, if the volume cannot be expanded (no outlet)
the pressure will increase approximately 700-fold or until it blows something
out!
Remedy: Every space in contact with the must have a pressure-relief device.
![]()