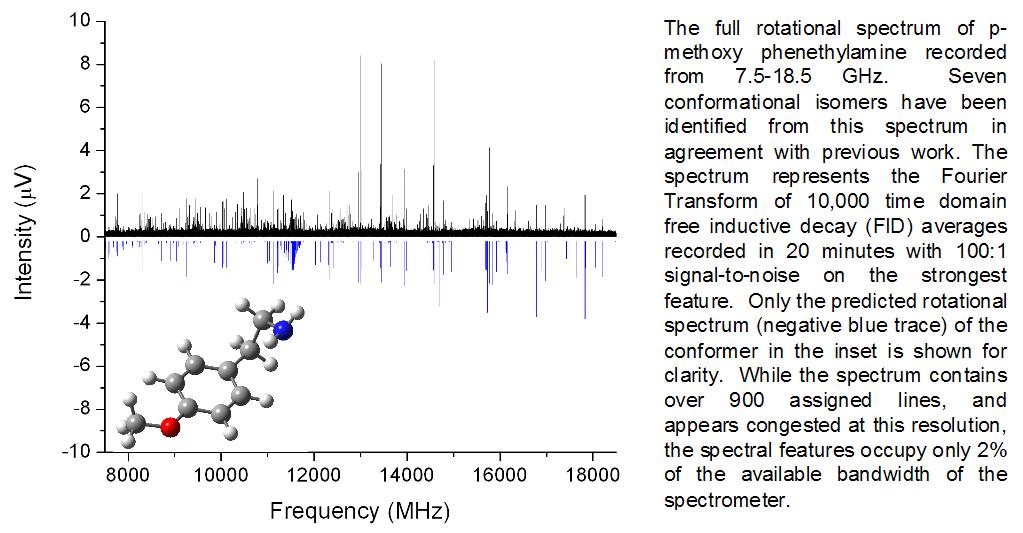
As molecular weight increases, characterization of combustion species becomes increasingly difficult. This difficulty stems from the fact that a single mass unit combustion product can have multiple isomers, thus complicating mass-based detection schemes and congesting IR and UV spectra. Counter to this, the rotational spectrum of a molecule is unique to its structure, resulting in a molecular fingerprint that can be used to identify several isomers of the same mass. We use Chirped-Pulsed Fourier Transform Microwave (CP-FTMW) spectroscopy, a newly developed microwave technique that records the rotational spectrum of molecular species in a single data-acquisition event, towards the study of combustion reactions.
This breakthrough in technology provides, for the first time “shape sensitive” detection. Initial experimental work will focus on applying CP-FTMW spectroscopy to studying “first ring” formation chemistry, a necessary pathway to soot formation in aliphatic combustion sources. The power of the technique lies in drastic reduction of data acquisition times. The capability of CP-FTMW to uniquely and rapidly identify isomers of the same mass complements current mass-based detection methods.
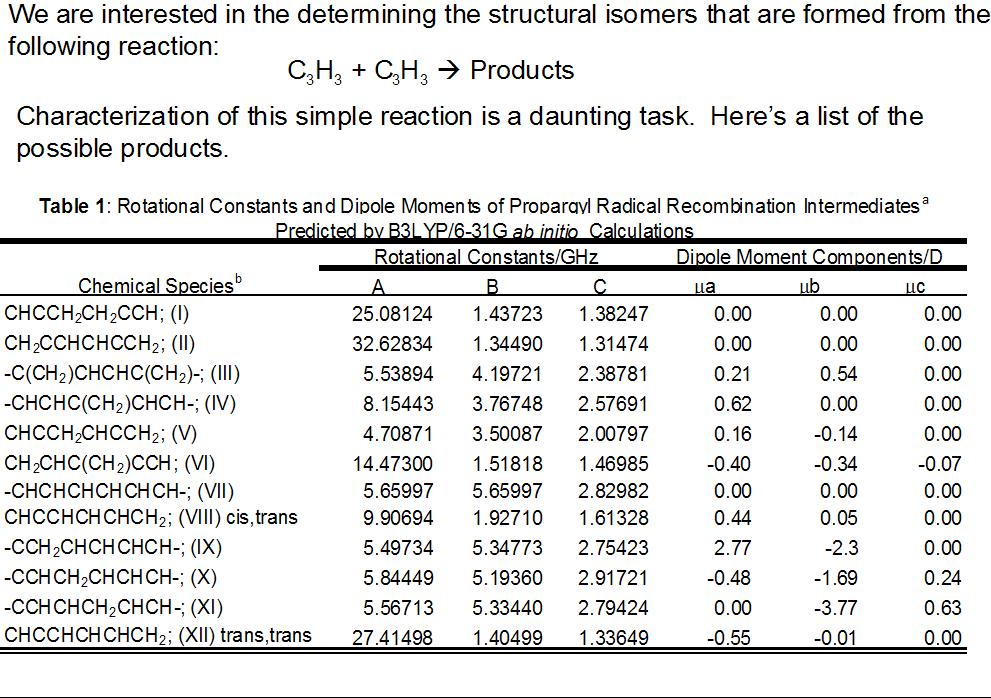
One of the advantages of rotational spectroscopy is that it provides detailed structural information about molecules. This is an example of how it can be used to determine conformational isomers (same chemical bonds), but it can also be used to determine structural isomers (different chemical bonds).
|
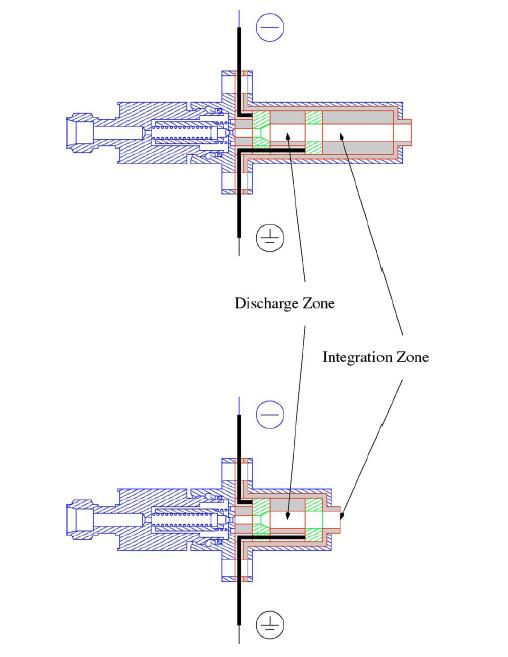
How do we initiate chemical reactions? We are currently working on building a pulsed discharge nozzle. This nozzle passes a high voltage discharge between two electrodes that ionizes the buffer gas (typically Ar). The ionized Ar will then make free radicals of the dilute chemical species (propargyl iodide) seeded into the buffer gas. Since these radicals are highly reactive, they combine with other radicals to create exotic chemical species that are difficult to make through a bench-top synthesis. By monitoring the rotational spectrum, we can detect the different products species that are formed. In fact, if all of the products have dipole moments, we can detect ALL of the products that are formed at once. This will give us information about the reaction surface that the radical species travel on their way to products. |
|
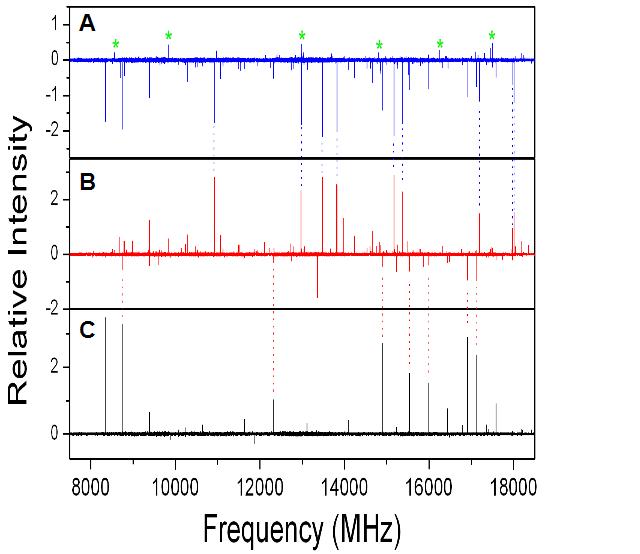
The rotational spectrum of D-erythrose taken at three different temperatures. At low temperatures (90 C, panel C) the spectrum is dominated by water dimer lines as water is boiled off of the sample. At higher temperatures (150 C, panel B) the water dimer lines are diminished and new lines grow-in, assigned to D-erythrose. Panel B is a difference spectrum of the 150 C and 90 C spectra showing the reduction of water dimer and the increase in D-erythrose lines. At the highest temperature (155 C; Panel A) the difference spectrum (155 C - 150 C) shows a further reduction in water dimer lines and D-erythrose, and an increase in new features (marked with asterisks) as the sample begins to decompose. A single rotational spectrum was recorded in approximately five minutes (1000 averages). The equivalent rotational spectrum recorded by conventional microwave spectrometers would take 14 hours. |