Our experimental approach relies on the ability to generate ultra-broadband (> 10 GHz) microwave pulses in short timescales (< 10 ns). We do this using state-of-the-art digital electronics. The devices we use to do this have only been commercially available for a couple of years. Here’s a picture of what they look like:

Why do we need ultra-broadband pulses? If we want to probe dynamics, we need the ability to probe different conformations of a single molecule simultaneously. These transitions can be GHz apart, therefore we need a broadband source to excite and detect the changes in different molecular structures.
The chemistry of highly excited molecules plays important roles in areas such as biological systems and combustion chemistry where the structure of these species is important in understanding their chemical nature. Modern understanding of chemical structure and dynamics relies heavily on spectroscopic techniques that can provide either detailed structural information to high precision, or dynamical information on femtosecond time-scales. However, many of the techniques currently used today are either/or devices that excel in either providing structural or dynamical information, but seldom both.
We use Chirped-Pulse Fourier transform microwave (CP-FTMW) spectroscopy to accomplish this feat. The power of this spectrometer is tied to its ability to record rotational spectra of any molecular species that has a permanent dipole moment. For the first time the rotational spectrum of a molecule can be used as a detector much like mass- or fluorescence-based detection techniques representing a quantum leap forward in rotational spectroscopy. This work involves pump-probe spectroscopy in which the visible or ultraviolet laser pulse initiates an event that is followed after relaxation via its full microwave spectrum as a function of delay between successive microwave pulses.
What do we mean by highly excited molecules?
These are molecules that have ~ 30,000 cm-1 of internal energy (remember that a hydride stretch fundamental has ~ 3000 cm-1 of internal energy). This is enough energy to make and break bonds. How do we do this? We use a laser to transfer ground state molecules to excited electronic states. Furthermore, since we are interested in dynamics, we want to know precisely how much energy the molecule has when it undergoes reaction. Since room temperature molecules have a lot of energy in them, we expand them in a vacuum to a few degrees Kelvin, essentially removing all internal energy from the molecules.

What kinds of problems are we interested in looking at? Initially we are looking at reactions that occur after excited state processes such as internal conversion and intersystem crossing. An example system is o-methylbenzaldehyde. A schematic of the potential energy surface is shown below. Only the cis form (1a) is oriented to undergo excited state proton transfer. In this way we can not only initialize, but also detect conformation specific chemistry
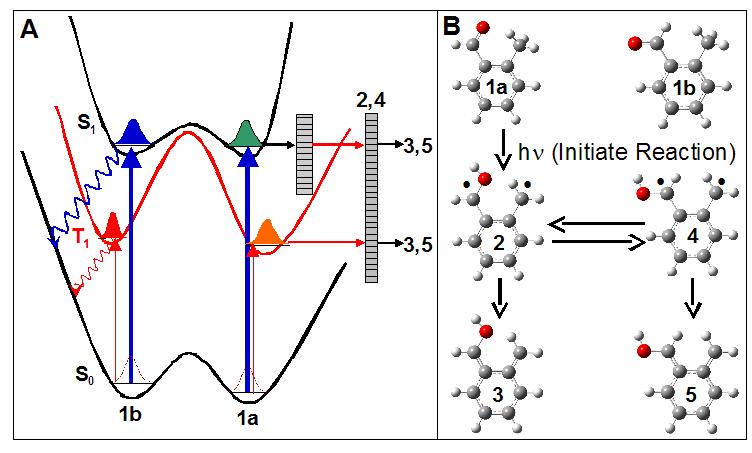
So why do we need microwaves to do this? The only requirement that a molecule needs to interact with a microwave field is that it has a permanent dipole moment. This has the advantage that molecules that are in “dark” states (so called because they interact very weakly with laser beams) can easily be detected with microwave pulses. What we use is called a “pump-probe” technique. We use the laser beam to pump the molecules full of energy and initiate the reaction. We can then probe the reaction with our microwave pulses.
This also highlights why we need fast microwave pulses. Chemical reaction can take place in microsecond or less. If we want to probe the individual steps of a reaction (so-called transition states) we need to be able to make microwave pulses that are shorter than the lifetime of the intermediate states.
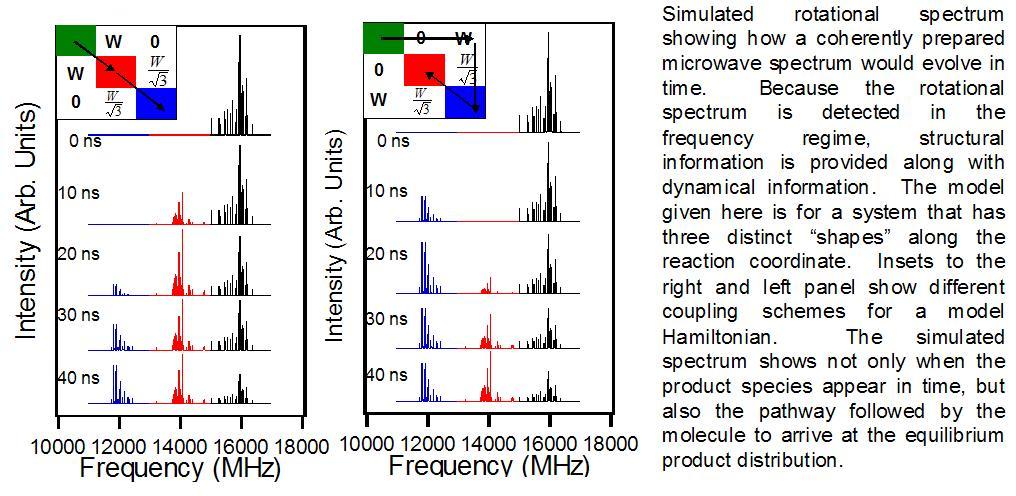
So where do we think these experiments will lead? Ultimately we want to follow a reaction as it proceeds in real time. Because we use the rotational spectrum of a molecule as a probe, we get structural information about the molecule for free. This will not only give us the time scale of a reaction, but also pathway information about how the molecule traverses the potential surface from reactants to products. Here’s a three step example: