![]()
Because of the desirability of constructing complex organic targets via confluent strategies, 6-7 carbon segments are often synthesized as logical sub-goals in preparation for assembly of the final targets. We are currently developing a unified synthetic strategy for the synthesis of highly-functionalized and stereochemically diverse 6-7 carbon backbone fragments. This approach emphasizes the creation of absolute stereochemistry via catalytic processes starting with inexpensive and easily prepared prochiral molecules. This strategy often delivers significantly enhanced efficiency when compared to syntheses based upon refunctionalization of chiral pool starting materials or procedures dependent upon stoichiometric utilization of chiral auxiliaries.
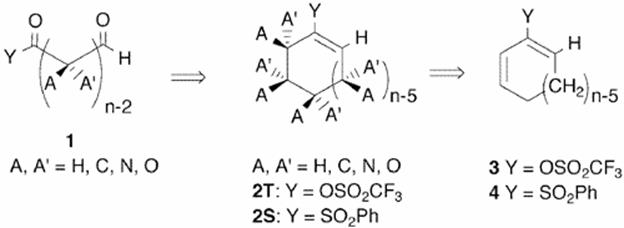
![]()
Recent publications:
| 186. | Chiral Carbon Catalog 2 Synthesis of 6-Carbon Termini-Differentiated Stereotriads via Symchiral 2-Trifloxy-1,3-cyclohexadiene monoepoxide. Hentemann,M.; Fuchs, P.L. Tetrahedron Lett. 1999, 40, 2699-2702. Jacobsen epoxidation of 2-trifloxy-1,3-cyclohexadiene provides a valuable asymmetric monoepoxide product that can be readily manipulated to efficiently provide highly-functionalized symchiral cyclic and acyclic synthons.
|
| 187. | Chiral Carbon Catalog 3 Synthesis of a Family of Epoxyvinyltriflate Stereotetrads from 4-Hydroxycyclohex-2-ene-1-one. Evarts, J.B., Jr.; Fuchs, P.L. Tetrahedron Lett. 1999, 40, 2703-2706. 4-Hydroxy cyclo-2-en-1-one can be converted into a family of highly oxygenated cyclohexyl epoxyvinyltriflates by epoxidation, rearrangement, and epoxidation. Furthermore, double stereoselection via Jacobsen epoxidation enables synthesis of compounds such as 20a which were previously very difficult to prepare.
|
| 190. | Chiral Carbon Catalog 4 Asymmetric Epoxy Cyclohexenyl Sulfones: Readily Accessible Progenitors of Stereodefined Six-Carbon Arrays. Hentemann, M.; Fuchs, P.L. Organic Lett. 1999, 1, 355-357. Enantiopure epoxy vinyl sulfones serve as highly effective substrates for a variety of stereo- and regiospecific oxidation and nucleophilic functionalization reactions. These materials can be easily transformed to cyclic and acyclic six-carbon segments. Nucleophilic epoxidation of 3a,b followed by palladium[0] catalysis enables access to differentially protected arene diols 21 and 22.
|
| 194. | Chiral Carbon Catalog 5 Synthesis of Enantiopure Termini-Differentiated Heptane Stereotriads. Jiang W.; Lantrip, D.A.; Fuchs, P.L. Organic Lett. 2000, 2, 2181-2184. Enantiopure epoxy cycloheptenyl sulfones syn-7b and anti-7b are prepared in five high-yielding and stereospecific operations from 1,3-cycloheptadiene. These substrates serve as effective precursors for cis- and trans-substituted tetrahydrofurans (12, 10) which are segments of the antineoplastic agent IKD-8344.
|
| 199. | Chiral Carbon Catalog 6 Economical and Environmentally Friendly Syntheses of 2-(Phenylsulfonyl)-1,3-cyclohexadiene and 2-(Phenylsulfonyl)-1,3-cycloheptadiene. Meyers, David J.; Fuchs, P.L. J. Org. Chem. 2002, 67, 200-204. A large-scale and inexpensive synthesis of dienes 1 and 2 has been developed via a four-step procedure starting with benzenethiol and the corresponding cyclic ketone. No chromatography is required.
|
| 204. | Chiral Carbon Catalog 7 Synthesis of Highly Substituted Enantiopure C6 and C7 Enones. Evarts, J,; Torres, E.; Fuchs, P.L. J. Am. Chem. Soc. 2002, 124, 11093-11101. Enantiopure epoxyvinyl sulfones SS-9A, SS-9b produced from Jacobsen epoxidation of 2-phenylsulfonyl 1,3-cyclohexa- and cycloheptadiene, are used as a template for the construction of substituted cycloalkenones and as chiral synthetic equivalents of enones a and b. The addition of carbon nucleophiles to SS-9a, SS-9b is highly yielding and stereospecific. Enantiopure alpha, beta, and gamma-substituted cycloalkenones are easily constructed using a variety of methods . |
![]()