![]()
At present, there are thirty known trisdecacyclic (thirteen rings) pyrazines which have been isolated from two very different marine organisms by the groups of Pettit at Arizona State University (cephalostatins 1-17) and Fusetani at the University of Tokyo (ritterazines A-M). In addition to the fascinating topology and biosynthetic origin of these compounds, fervent interest centers around their outstanding potential as antineoplastic agents. Cephalostatin 1 is the most potent inhibitor of the family with an ED50 10-7-10-9 mg/mL in the P38 cell line, and cephalostatin 1 and 7 are desired for Phase I clinical trials in Europe.
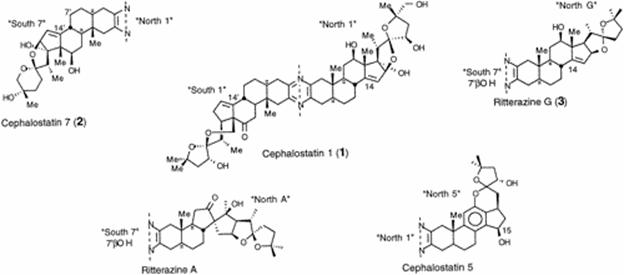
![]()
Recent publications:
| 212. | CSTAT 29 Polyphosphoric Acid Trimethylsilyl Ester Promoted Intramolecular Acylation of an Olefin by a Carboxylic Acid: Convenient Construction of C-18 Functionalized D14-Hecoginin Acetate. Li, W.; Fuchs, P.L. Organic Lett. 2003, 5, 4061-4064. Polyphosphoric acid trimethylsilyl ester (PPSE)-promoted intramolecular Friedel-Crafts reactions on a nonaromatic carboxylic acid system have been investigated. Studies led to the synthesis of C-18 functionalized steroidal compounds 5 and 9a-d with strict retention of the spiroketals. Isomerization of spiroketal 9e was studied.
|
| 213. | CSTAT 30 Trifluoroacetyl Trifluoromethanesulfonate (TFAT): An Efficient Agent for Ring Opening of Spiroketals. Lee, J.S.; Fuchs, P.L., Organic Lett. 2003, 5, 3619-3622. Ring opening of steroidal spiroketals under exceptionally mild conditions is smoothly achieved via reaction with trifluoroacetyl trifluoromethanesulfonate (TFAT). The new spiroketal ring-opening protocol provides omega-trifluoroacetyl vinyl ethers in good yield and avoids difficulties that attended previously employed vigorous reaction conditions.
|
| 216. | CSTAT 31 An Efficient C-H Oxidation Protocol for the alpha-Hydroxylation of Cyclic Steroidal Ethers. Lee, S.M.; Fuchs, P.L. Organic Lett. 2004, 6, 1437-1440. Various C-16 hydroxy steroids have been prepared with the aid of CrO3/Bu4NIO4. Out of the two possible reaction courses, transition state B is favored because of less steric interference between substrate and CrO4. Thus, C-H bonds at C-16 are oxidized selectively.
|
![]()