

Monodentate ligands are Lewis bases that donate a single pair ("mono") of electrons to a metal atom. Monodentate ligands can be either ions (usually anions) or neutral molecules.
| Some Monodentate Ligands | |||||
|---|---|---|---|---|---|
| ligand | Lewis structure | name | ligand | Lewis structure | name |
| F- | 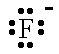 | fluoride ion | Cl- | 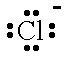 | chloride ion |
| Br- | 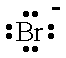 | bromide ion | I- | 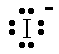 | iodide ion |
| H2O |  | water | NH3 | 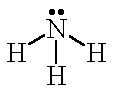 | ammonia |
| OH- | 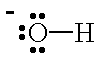 | hydroxide ion | CO | carbon monoxide | |
| CN- | cyanide ion | SCN- | 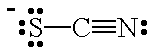 | thiocyanate ion |
Chemists often represent ligands as spheres for simplicity, even though the "sphere" sometimes has three-dimensional structure of its own. For example, when chemists draw the structure for [Ni(NH3)6]2+, each ammonia ligand is represented as a sphere. The sphere represents the donor atom of the ligand. In [Ni(NH3)6]2+, the donor atoms are the nitrogen atoms of the NH3 ligands (NOT the hydrogen atoms).
|
|
||
| [Ni(NH3)6]2+ abbreviated structure Note: only the donor atoms (N atoms) of the NH3 ligands are shown. |
[Ni(NH3)6]2+ complete structure Note: all atoms are shown. |
|
|
||
| [Cu(NH3)4]2+ Used in some brands of waterbed conditioners to inhibit the growth of fungi and bacteria. Note the square planar structure. |
[RhI2(CO)2]- Used as a catalyst in the Monsanto Process for making acetic acid. Note the square planar structure. |
