
Calculating the Reaction Quotient, Q
The expression for the reaction quotient, Q, looks like that used to calculate an equilibrium constant but Q can be calculated for any set of conditions, not just for equilibrium.
Q can be used to determine which direction a reaction will shift to reach equilibrium. If K > Q, a reaction will proceed forward, converting reactants into products. If K < Q, the reaction will proceed in the reverse direction, converting products into reactants. If Q = K then the system is already at equilibrium.
In order to determine Q we need to know:
- the equation for the reaction, including the physical states,
- the quantities of each species (molarities and/or pressures), all measured at the same moment in time.
- Write the expression for the reaction quotient.
- Find the molar concentrations or partial pressures of each species involved.
- Subsitute values into the expression and solve.
SO2Cl2(g) ![]() SO2(g) + Cl2(g)
Kc = 0.078 at 100oC
SO2(g) + Cl2(g)
Kc = 0.078 at 100oC
-
Write the expression to find the reaction quotient, Q.
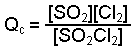
-
Since Kc is given, the amounts must be expressed as moles per
liter (molarity).
The amounts are in moles so a conversion is required.
0.500 mole SO2Cl2/5.00 L = 0.100 M SO2Cl2 0.035 mole SO2/5.00 L = 0.070 M SO2 0.080 mole Cl2/5.00 L = 0.016 M Cl2 -
Substitute the values in to the expression and solve
for Q.
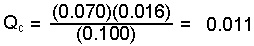
- Compare the answer to the value for the equilibrium constant and predict the shift.
Since K >Q, the reaction will proceed in the forward direction in order to increase the concentrations of both SO2 and Cl2 and decrease that of SO2Cl2 until Q = K.
