

A liquid boils at a temperature at which its vapor pressure is equal to the pressure of the gas above it. The lower the pressure of a gas above a liquid, the lower the temperature at which the liquid will boil.
The Macroscopic View
The boiling point of a liquid is the temperature at which its vapor pressure is equal to the pressure of the gas above it.The normal boiling point of a liquid is the temperature at which its vapor pressure is equal to one atmosphere (760 torr).
The Microscopic View
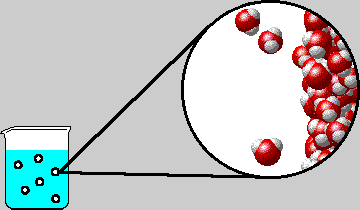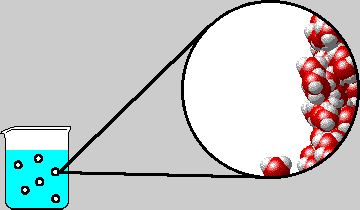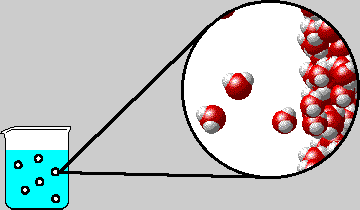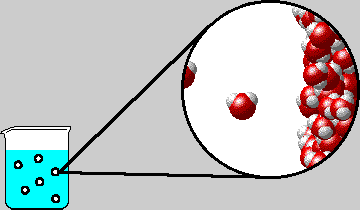 |
| Microscopic view inside a bubble in boiling water. The diagram shows the right-hand inner surface of the bubble. Note that water gas and liquid are in equilibrium. |
The following graph shows the boiling point for water as a function of the external pressure. The line on the graph shows the normal boiling point for water.
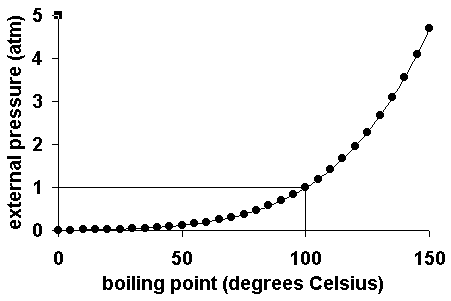
|
|
||
| methyl ether (C2H6O) The normal boiling point of methyl ether is -25oC (i.e., a gas at room temperature). The relatively weak dipole-dipole forces and London dispersion forces between molecules results in a much lower normal boiling point compared to ethyl alcohol. |
ethyl alcohol (C2H6O) The normal boiling point of ethyl alcohol is 78.5oC (i.e., a liquid at room temperature). Although dipole-dipole forces and London dispersion forces also exist between ethyl alcohol molecules, the strong hydrogen bonding interactions are responsible for the much higher normal boiling point compared to methyl ether. |
