

Hydrogen bonding is a special type of dipole-dipole attraction between molecules, not a covalent bond to a hydrogen atom. It results from the attractive force between a hydrogen atom covalently bonded to a very electronegative atom such as a N, O, or F atom and another very electronegative atom. Hydrogen bond strengths range from 4 kJ to 50 kJ per mole of hydrogen bonds.
| element |
|
| H | 2.1 |
| N | 3.0 |
| O | 3.5 |
| F | 4.1 |
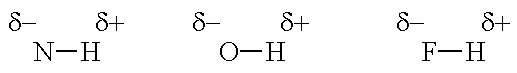
|
|
||
| Hydrogen bonding between two water (H2O) molecules. Note that the O atom in one molecule is attracted to a H atom in the second molecule. | Hydrogen bonding between a water molecule and an ammonia (NH3) molecule. Note that the N atom in the NH3 molecule is attracted to a H atom in the H2O molecule. |
Physical Consequences of Hydrogen Bonding
|
|
||
| The structure of ONF. | The structure of H2O. | |
 |
 |
|
| Microscopic view of ONF at 25oC. | Microscopic view of H2O at 25oC. |
