
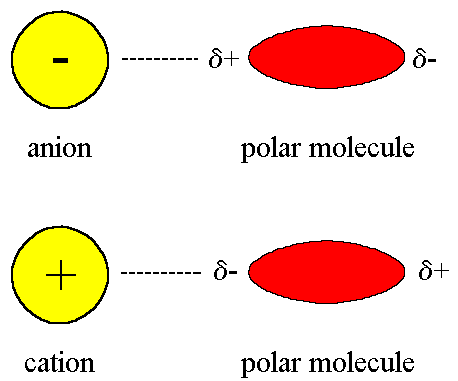
Ion-Dipole Forces
An ion-dipole force is an attractive force that results from the electrostatic
attraction between an ion and a neutral molecule that has a dipole.
-
Most commonly found in solutions. Especially important for solutions of
ionic compounds in polar liquids.
-
A positive ion (cation) attracts the partially negative end of a neutral
polar molecule.
-
A negative ion (anion) attracts the partially positive end of a neutral
polar molecule.
-
Ion-dipole attractions become stronger as either the charge on the ion
increases, or as the magnitude of the dipole of the polar molecule increases.




