

Surface tension is measured as the energy required to increase the surface area of a liquid by a unit of area. The surface tension of a liquid results from an imbalance of intermolecular attractive forces, the cohesive forces between molecules:
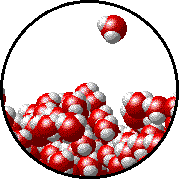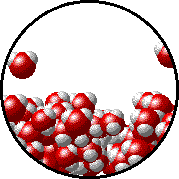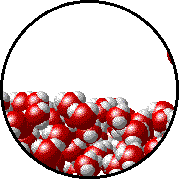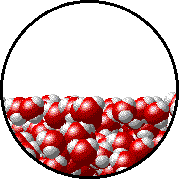
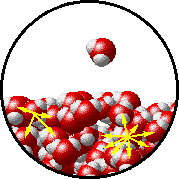
A microscopic view of water illustrates the difference between molecules at the surface of a liquid and water molecules within a liquid.
 |
 |
|---|---|
| The molecules at the surface of this sample of liquid water are not surrounded by other water molecules. The molecules inside the sample are surrounded by other molecules. | The unbalanced attraction of molecules at the surface of a liquid tends to pull the molecules back into the bulk liquid leaving the minimum number of molecules on the surface. It required energy to increase the surface area of a liquid because a larger surface area contains more molecules in the unbalanced situation. |
|

|
| When liquid water is confined in a tube, its surface (meniscus) has a concave shape because water wets the surface and creeps up the side. | Mercury does not wet glass - the cohesive forces within the drops are stronger than the adhesive forces between the drops and glass. When liquid mercury is confined in a tube, its surface (meniscus) has a convex shape because the cohesive forces in liquid mercury tend to draw it into a drop. |
