

The concentration of a solution
Qualitative Expressions of Concentration
A solution can be qualitatively described as
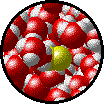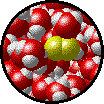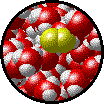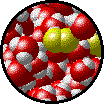 |
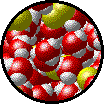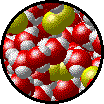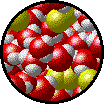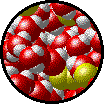 |
| Microscopic view of a dilute solution of liquid Br2 dissolved in liquid water. | Microscopic view of a concentrated solution of liquid Br2 dissolved in liquid water. |
Semi-Quantitative Expressions of Concentration
A solution can be semi-quantitatively described as
The solubility of a solute is the amount of solute that will dissolve in a given amount of solvent to produce a saturated solution. For example, at 0oC, we can dissolve a maximum of 35.7 g of solid NaCl in 100 mL of water (a saturated solution). Any additional solid NaCl that we add to the saturated solution simply falls to the bottom of the container and does not dissolve.
Quantitative Expressions of Concentration
There are a number of ways to express the relative amounts of solute and solvent in a solution. Which one we choose to use often depends on convenience. For example, it is sometimes easier to measure the volume of a solution rather than the mass of the solution.
Note that some expressions for concentration are temperature-dependent (i.e., the concentration of the solution changes as the temperature changes), whereas others are not. This is an important consideration for experiments in which the temperature does not remain constant.
| Temperature Dependence of Several Concentration Expressions | ||
|---|---|---|
| concentration expression | measurements required | temperature dependent? |
| percent composition (by mass) |
mass of solute mass of solution |
no (mass does not change with temperature) |
| molarity | moles of solute volume of solution |
yes (volume changes with temperature) |
| molality | moles of solute mass of solvent |
no (neither mass nor moles changes with temperature) |
| mole fraction | moles of solute moles of solvent |
no (moles does not change with temperature) |
Percent Composition (by mass)
We can consider percent by mass (or weight percent, as it is sometimes called) in two ways:
Use the following equation to calculate percent by mass:
Molarity
Molarity tells us the number of moles of solute in exactly one liter of a solution. (Note that molarity is spelled with an "r" and is represented by a capital M.)
We need two pieces of information to calculate the molarity of a solute in a solution:
To calculate molarity we use the equation:
Molality
Molality, m, tells us the number of moles of solute dissolved in exactly one kilogram of solvent. (Note that molality is spelled with two "l"'s and represented by a lower case m.)
We need two pieces of information to calculate the molality of a solute in a solution:
To calculate molality we use the equation:

Mole Fraction
The mole fraction, X, of a component in a solution is the ratio of the number of moles of that component to the total number of moles of all components in the solution.
To calculate mole fraction, we need to know:
The mole fraction of A, XA, in a solution consisting of A, B, C, ... is calculated using the equation:

To calculate the mole fraction of B, XB, use:

