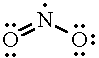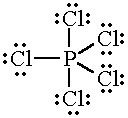Counting Regions of High Electron Density
- Draw the Lewis structure for the molecule or ion.
- Count the total number of regions of high electron density (bonding and unshared electron pairs) around the central atom.
- Double and triple bonds count as ONE REGION OF HIGH ELECTRON DENSITY.
- An unpaired electron counts as ONE REGION OF HIGH ELECTRON DENSITY.
- For molecules or ions that have resonance structures, you may use any one of the
resonance structures.
| molecule | Lewis
structure | # regions of high electron density | molecule | Lewis
structure | # regions of high electron density |
| BeCl2 |
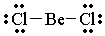 |
2
|
BF3 |
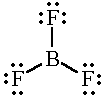 |
3
|
| HCN |
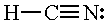 |
2
|
SO3 |
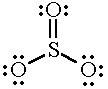 |
3
|
| CO2 |
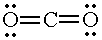 |
2
|
NO2 |
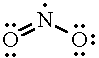 |
3
|
| CH4 |
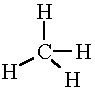 |
4
|
NH3 |
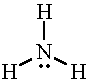 |
4
|
| PCl5 |
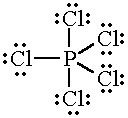 |
5
|
SF6 |
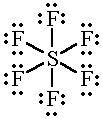 |
6
|
 VSEPR Rules
VSEPR Rules