

Because water can function as a Lewis base and transition metal ions can function as Lewis acids, most salts containing transition metals form coordination complexes when they are dissolved in water.
All salts which contain a first-row transition metal (i.e., Sc, Ti, V, Cr, Mn, Fe, Co, Ni, Cu, Zn) form octahedral complex ions when they are dissolved in water.
In aqueous solution, transition metal cations are usually symbolized as Mn+(aq), where M is the atomic symbol of the metal ion and n is the charge on the ion. For example, Fe3+ in aqueous solution is written as Fe3+(aq). The (aq) symbol indicates that the metal ion is aquated (i.e., the metal ion is bonded to several water molecules).
|
|
||
| [Fe(OH2)6]3+ The octahedral species produced when solid Fe(NO3)3 is dissolved in water (NO3- ions are not shown). Note that this species is also written as: Fe3+(aq). |
[Co(OH2)6]2+ The octahedral species produced when solid Co(NO3)2 is dissolved in water (NO3- ions are not shown). Note that this species is also written as: Co2+(aq). |
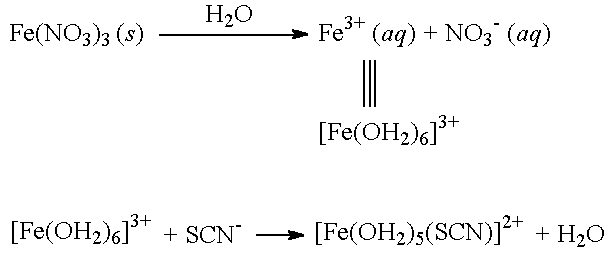
In aqueous solution, [Fe(OH2)6]3+ ions react with SCN- (not free Fe3+ ions). In the reaction shown above, the SCN- ion replaces a water molecule in the coordination sphere to produce the complex ion, [Fe(OH2)5(SCN)]2+.
