

Geometric isomers are two or more coordination compounds which contain the same number and types of atoms, and bonds (i.e., the connectivity between atoms is the same), but which have different spatial arrangements of the atoms.
Not all coordination compounds have geometric isomers.
For example, in the square planar molecule, Pt(NH3)2Cl2, the two ammonia ligands (or the two chloride ligands) can be adjacent to one another or opposite one another.
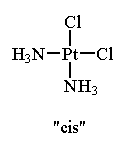 | 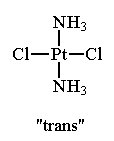 |
Note that these two structures contain the same number and kinds of atoms and bonds but are non-superimposable. The isomer in which like ligands are adjacent to one another is called the cis isomer. The isomer in which like ligands are opposite one another is called the trans isomer.
|
|
||
| cis-Pt(NH3)2Cl2 Note that the two NH3 ligands and the two Cl ligands are adjacent to one another. This compound is used medicinally to treat certain types of cancer. (tradename: "cisplatin"). |
trans-Pt(NH3)2Cl2 Note that the two NH3 ligands and the two Cl ligands are opposite one another. This compound does not exhibit any anti-tumor activity. |
For the common structures which contain two or more different ligands, geometric isomers are possible only with square planar and octahedral structures (i.e., geometric isomers cannot exist for linear and tetrahedral structures).
|
|
||
| cis-[Co(NH3)4Cl2]+ Note that the two chloride ligands are adjacent to one another in this octahedral complex ion. In aqueous solution, this complex ion has a violet color. |
trans-[Co(NH3)4Cl2]+ Note that the two chloride ligands are opposite one another in this complex ion. In aqueous solution, this complex ion has a green color. |
