

Some ligands can bond to a metal atom using more than two pairs of electrons. An example is ethylenediamminetetraacetate ion (EDTA4-), the Lewis structure of which is shown below. EDTA4- forms very stable complexes with most of the transition metals.
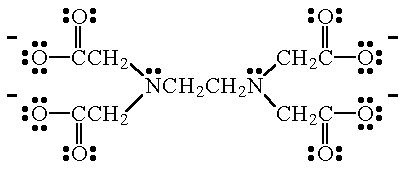 |
| EDTA4- This hexadentate ligand forms very stable complexes (usually octahedral structures) with most of the transition metals. The donor atoms in EDTA4- are the two N atoms, and the four, negatively charged O atoms. |
|
|
||
| EDTA4- When this ion bonds to a metal atom, the two N atoms, and four of the O atoms, are used. |
[Fe(EDTA)]2- In this complex, a single EDTA4- ion forms 6 bonds to the Fe atom (i.e., 2 Fe-N bonds and 4 Fe-O bonds). The coordination number of 6 results in an octahedral structure. |
EDTA4- is used to "trap" trace amounts of transition metals that could potentially catalyze the decomposition of the product.
