Theoretical Chemistry Purdue
Slipchenko Group
Electronic excitations in the condensed phase
The environment alters chemical processes by perturbing electronic wave functions, changing relative energetics of polar and non-polar states/species, affecting couplings between electronic states, and by confining effects (caging). In extreme cases, a solvent may completely change the character of the electronic states of a solute and create new states that do not exist in the gas phase (e.g., so called charge-transfer-to-solvent states). Our goal is to understand how the environment affects electronic states and interactions (couplings) between them and to connect computational findings with spectroscopic observables. A series of excited-state QM/MM techniques in which the MM part is described by EFP that we developed allow us to pursue this goal.
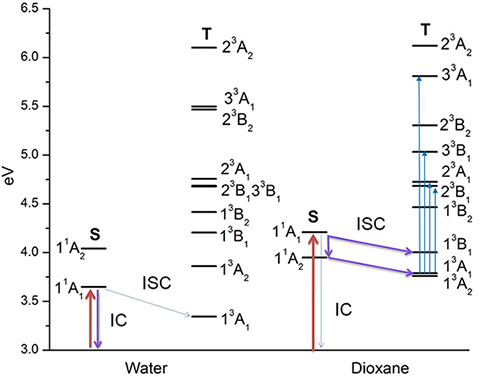
Singlet and triplet states of para-nitroaniline solvated in water (left) and dioxane (right) and energy relaxation channels: internal conversion (IC) and intersystem crossing (ISC).
Solvatochromic shifts in para-nitroaniline
Ionization energy in hydrated thymine
Review of QM/EFP for excited states