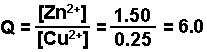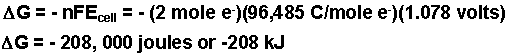Equilibrium Constant and Free
Energy Change
for an Electrochemical Cell
Two important important parameters that can be determined from a cell potential are the equilibrium constant for the cell reaction and the free energy change for the cell reaction.
- Determining the Equilibrium Constant from Eocell
- Determining the Standard State Free Energy Change from Eocell
- Determining the Non-Standard Free Energy Change
To calculate the equilibrium constant for an electrochemical cell we need to know:
- the standard state potential for a cell
- the half-reactions involved
![]()
At equilibrium Q = K. Substituting in K for Q, and the values for R, T, and F, we get:
![]()
Example: Find the value of the equilibrium constant at 25oC for the cell reaction for the following electrochemical cell:
Cu | Cu2+(1 M) || Ag+(1 M) | Ag.
- Write the equations for the cell half-reactions, calculate the standard cell potential, and determine the number of electrons transferred.
| 2 Ag+(aq) + 2 e- |
Eoreduction = + 0.799 V |
| Cu(s) |
Eooxidation = - 0.518 V |
|
|
|
| 2 Ag+(aq) + Al(s) |
Eocell = + 0.281 V |
n = 2 moles of electrons
- Substitute into the above equation and solve for K.
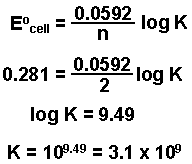
Note: values for the equilibrium constant for electrochemical cell reactions are sometimes very large.
Determining the Standard State Free Energy Change from Eocell
To determine the standard state free energy change for a cell reaction
- determine the Eocell
- determine the number of moles of electrons transfered in the reaction.
- solve for DGo using the equation
DGo
= standard state free energy change (joules)
n = number of moles of electrons
transferred
F = Faraday's constant (96,485
C/mol e-)
Eocell
= standard state cell potential (volts or joules/C)
Example: Find the value of the equilibrium constant at 25oC for the cell reaction for the following electrochemical cell:
Cu | Cu2+(1 M) || Ag+(1 M) | Ag.
(The solution for the determination of the Eocell and the number of moles of electrons, n, are shown in the example in the previous section. Click HERE to see the solution.)
- Determine the Eocell.
- Determine the number of moles of electrons transfered.
- Substitute into the equation and solve.
DGo = - 54,200 J or - 54.2 kJ
Determining the Non-Standard State Free Energy Change
To determine the non-standard state free energy change:
- calculate the standard cell potential, Eocell
- determine the number of moles of electrons transferred, n
- calculate the reaction quotient, Q
- calculate the non-standard cell potential, Ecell, using the Nernst equation
- Calculate the non-standard free energy change using the equation:
Example: Calculate the free energy change for the following electrochemical cell.
Zn(s) | Zn2+ (1.50 M) || Cu2+ (0.25 M) | Cu(s)
- Calculate Eocell.
- Determine "n".
- Calculate Q
- Calculate Ecell
- Calculate DG.
| Zn(s) |
Eooxidiation = + 0.762 volts |
| Cu2+(aq) + 2 e- |
Eoreduction = + 0.339 volts |
|
|
|
| Zn(s) + Cu2+(aq) |
Eocell = + 1.101 volts |