
Equilibria of Weak Acids, Ka
- What is a weak acid?
- Calculating Ka
- Calculating equilibrium concentraions in an aqueous solution of a weak acid
A weak acid is any acid that reacts with water (donates H+ ions) to a very small extent, usually less than 5 - 10%. An aqueous solution of a weak acid in a state of equilibrium would consist mainly of the unionized form of the acid, and only a small amount of hydronium ions and of the anion (conjugate base) of the weak acid. The equation representing the ionization of any weak acid, HA, and the equilibrium expression, Ka, are shown below.
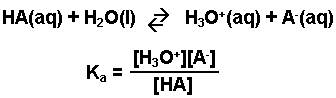
Note: This expression, Ka, is based on the general form for Kc. The designation Ka is used to indicate that it is the equilibrium constant for the reaction of an acid with water.
To calculate the ionization constant, Ka, you need to know:
- the equation for the reaction of the acid with water
- the equilibrium expression, Ka, for the reaction
- the equilibrium concentration of each species (molarity or moles per liter) or have a means to obtain them
Example: The pH of a 0.1000 M solution of acetylsalicylic acid (aspirin-"HAsp") was found to be 2.24. Determine the value of Ka, the ionization constant for acetylsalicylic acid, Ka. The formula for acetylsalicylic acid is CH3CO2C6H4COOH, but we use "HAsp" as an abbreviation.
-
Write the equation for the equilibrium between aspirin and water.
HAsp(aq) + H2O(l)
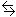 H3O+(aq)
+ Asp-(aq)
H3O+(aq)
+ Asp-(aq) -
Write the equilibrium expression for the reaction.
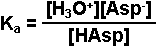
-
Convert the pH of the solution into the hydronium ion
concentration. This will be the equilibrium concentration of the
hydronium ion.
[H3O+] = 10-pH = 10-2.24 = 0.0057 M
-
Make an ICE chart to aid in identifying the
variables. The hydronium ion concentration in pure water is 1 x 10-7 M which can be considred as being
approximately zero.
HAsp(aq) H3O+(aq) Asp-(aq) Initial Concentration (M) 0.1000 ~ 0 0 Change in Concentration (M) - 0.00575 + 0.0057 + 0.0057 Equilibrium Concentration (M) 0.0943 0.0057 0.0057 For explanation of the significance of the different colors click HERE.
- Substitute the equilibrium concentrations into the equilbrium expression and solve for Ka.
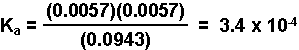
Calculating the Equilibrium Concentrations in an Aqueous Solution of a Weak Acid
To calculate the equilibrium concentrations you need to know:
- The equation for the reaction of the acid with water.
- The equilibrium expression for the reaction and the value of the equilibrium constant, Ka.
- The initial concentration of the acid expressed in moles per liter.
- Make an ICE chart. Express the change in equilibrium concentrations for each species in terms of a variable "x".
- Substitute the expressions for the equilibrium concentration into the equilibrium expression and solve for "x".
- Calculate the equilibrium concentration for each species by substituting in value for "x" and solving.
-
Write the equation for the reaction of the acid with
water.
HNO2(aq) + H2O(l)
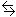 H3O+(aq)
+ NO2-(aq)
H3O+(aq)
+ NO2-(aq) -
Write the equilibrium expression for the reaction.
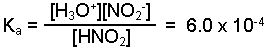
-
Make an ICE chart to aid in the solution of
the problem. Let "x" represents the change in concentration of H3O+ and NO2-.
HNO2(aq) H3O+(aq) NO2-(aq) Initial Concentraion (M) 0.1000 ~ 0 0 Change in Concentration (M) - x + x + x Equilibrium Concentration (M) 0.1000 - x 0 + x 0 + x For explanation of the significance of the different colors click HERE.
-
Substitute the expressions for the equilibrium concentration into the equilibrium
expression and solve for "x".
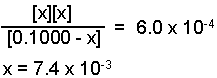
Solved using method of approximations or the quadratic equation.
-
Calculate the equilibrium concentration for each
species.
[H3O+] = x = 7.4 x 10-3M
[NO2-] = x = 7.4 x 10-3M
[HNO2] = 0.1000 - x = 0.0926 M
