

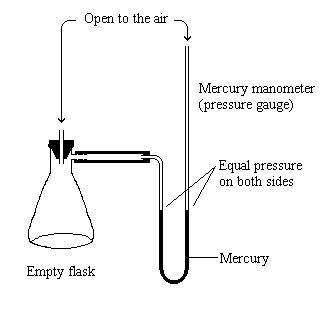
| The mercury on both sides of the manometer is at the same height because the pressure on both sides is equal. |
 |
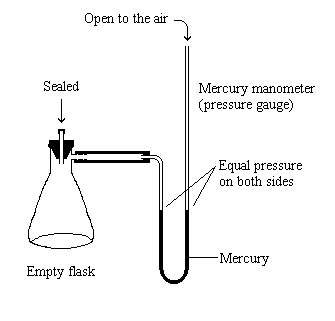
| The flask is capped carefully so that the pressure on both sides remains equal. |
 |
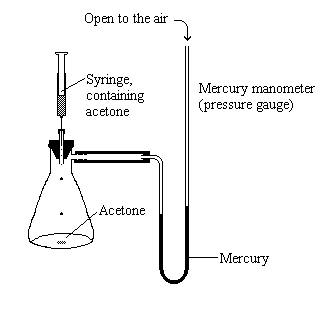
| As a little acetone is injected into the sealed flask the pressure in the flask begins to increase as the acetone evaporates. |
 |
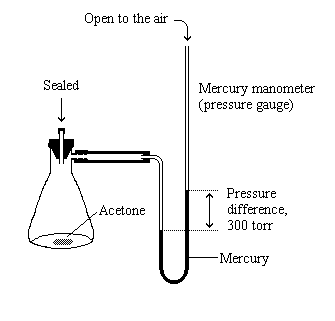
| The 300 torr increase in pressure is due to the evaporation of the
liquid in the flask. The vapor pressure of the liquid is equal to
the increase in pressure after the pressure stops changing.
Because the amount of liquid and vapor have stopped changing, we say the liquid and vapor are in equilibrium at this point. |
 |
