 The McLuckey Lab
The McLuckey Lab

Home-built Instrumentation
Our ongoing activities in novel tool development is supported by the Jonathan Amy Facility of Chemical Instrumentation (JAFCI). It is with the essential support of JAFCI, as well as our industrial collaborators, that we are able to innovate in instrumentation in an academic setting with graduate students spearheading the effort.
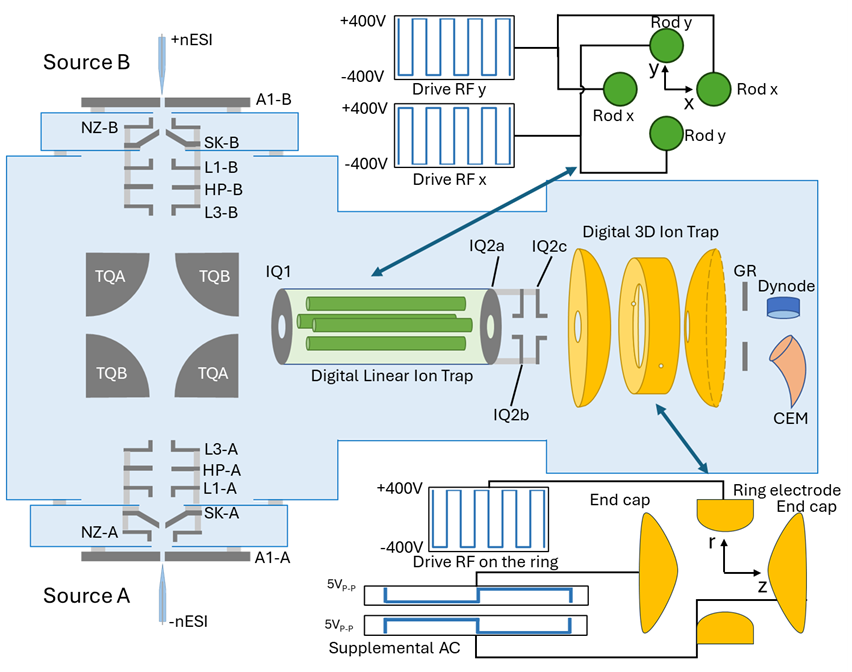
One of our homebuilt instruments is a tandem in space 2D/3D quadrupole ion trap mass spectrometer with IRMPD and UVPD capabilities. The most unique aspect of this instrument is the ability to use a different trapping waveform. Historically, a sinusoidal waveform is applied to the trapping electrodes, but here we can apply a digital (or square) waveform. The digital waveform allows us to more effectively trap and isolate large ions with lower voltages. This, combined with the tandem in space quadrupoles, allows us to probe large protein ions with our different ion/ion chemistries such as multiple ion attachment (MIA) and ion parking.

The Electrostatic Linear Ion Trap (ELIT) is a lab-built instrument focused primarily on training graduate students in mass spectrometry hardware development and through demonstrative application. Projects often involve extensive collaborative effort from electronics, hardware and instrumentation experts in the Purdue University Jonathan Amy Facility for Chemical Instrumentation (JAFCI), with many components being developed specifically for this instrument. The ELIT has undergone many configurations for specific projects but is currently configured with a trapping quad, for ion accumulation, positioned in front of a linear trap used for selective ion isolation and trapping. Previous experiments have included mirror switching for nonadjacent m/z ion isolation experiments, increasing ion resolution and mass range through multiple iterations of trap sizes, and activation experiments including surface induced dissociation of high-mass complex ions.
Click here to view a retrospective of all of the custom instrumentation projects performed by the McLuckey lab over the years. In here, you will find the first ESI-coupled ion trap, the first instruments designed for ion-ion reactions, and many others!
Commercially Modified Instruments
SCIEX 4000 QTrap
Our SCIEX 4000, modified to enable mutual storage of oppositely charged ions, has enabled the study of many different reactions to probe the structures and chemical properties of many biologically relevant analytes. Another key feature is the implementation of dual-spray nano-ESI emitters as well as software to control the accumulation of oppositely-charged ions in series. The use of separate emitters allows us to inject samples that can come from very different solution conditions. We have also developed an in-house APCI source that can be interfaced with another nESI emitter. External waveform generators give us the ability to apply complex and tailored waveforms for use in isolation, ion parking, and ion activation.
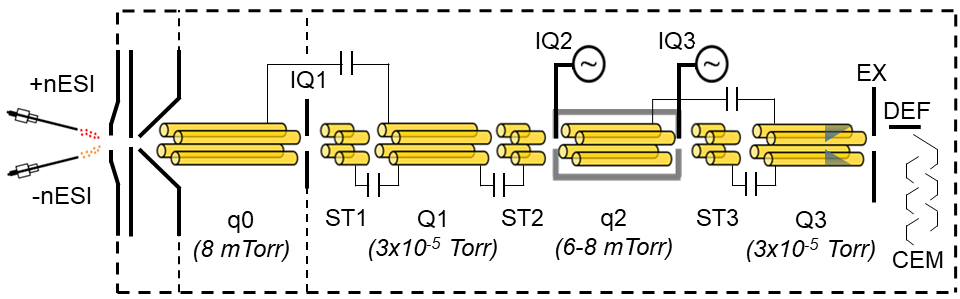
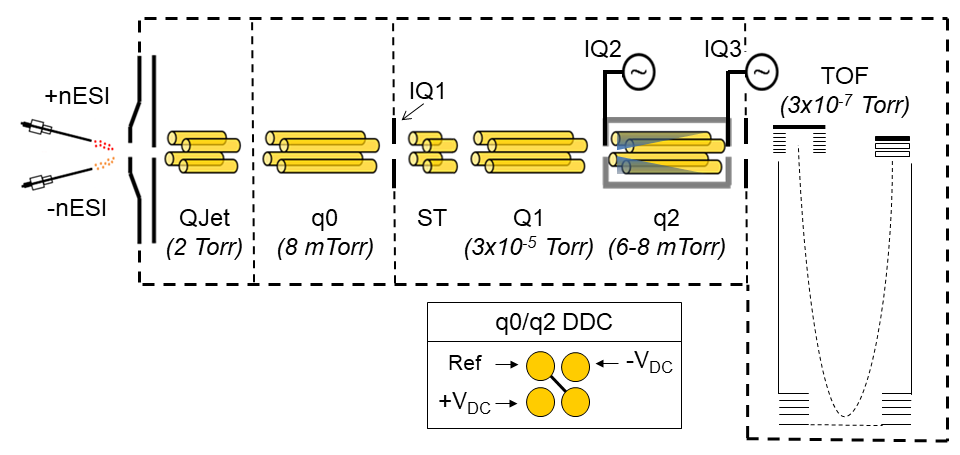
SCIEX 5600 QTOF
Our SCIEX 5600 is also capable of mutual storage of oppositely charged ions, dual spray nESI, and dipolar direct current (DDC) activation capabilities. It is also possible to adjust the time-of-flight pulser frequency, which allows detection of ions up to m/z 400,000. DDC is a broadband activation method that, unlike beam-type CID, occurs while ions are trapped. It is a slow heating method that, by way of the Tolmachev model, can yield thermodynamic information about analytes of interest. We can use DDC to control the kinetics of ion-ion reactions and the extent of ion cloud overlap radially. The linear accelerator (LINAC) can achieve the same effect axially. This gives us unparalleled control of the selectivity in reagents (via prior ion isolation) as well as the reaction kinetics.
Ion/Ion Chemistry and Its Applications to Biomolecules
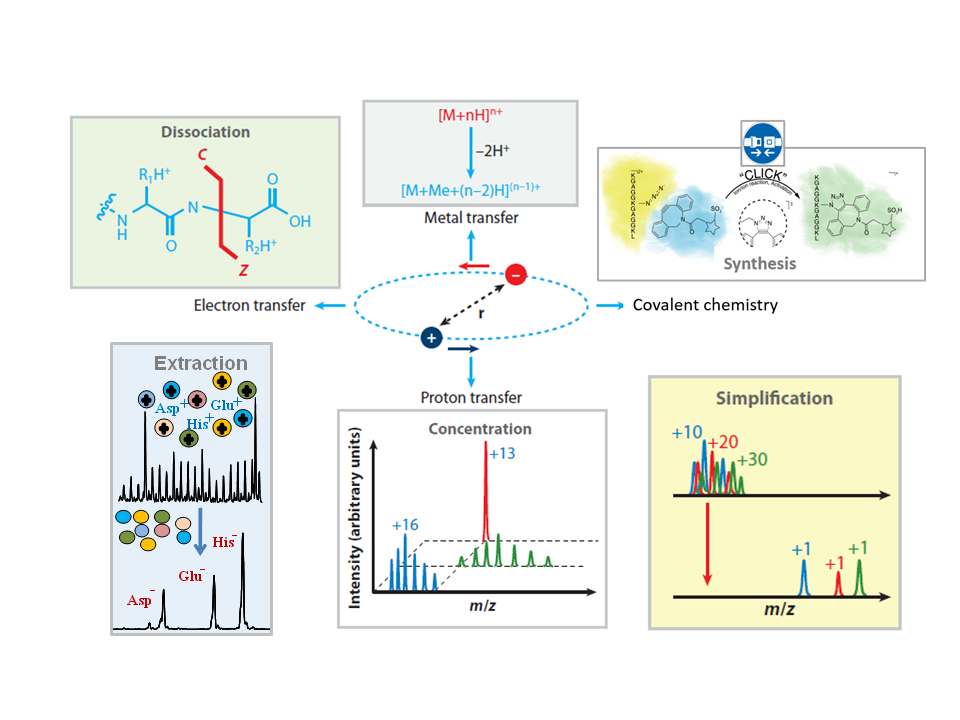
Gas-phase ion/ ion reactions constitute a signature area of R&D in this lab. Gas-phase ion-ion reactions are fast, efficient, and readily amenable to computer control. Ion isolation technologies allow for a high degree of selectivity in the identities of reactants and a wide array of reaction types can occur (e.g., proton transfer, electron transfer, covalent bond formation, ion attachment, charge inversion, etc.). The advent of electrospray ionization (ESI), which enables the generation of multiply-charged ions of either polarity, and electrodynamic ion traps, which enable the storage of ions of both polarities in overlapping regions of space, enable the study of ion-ion reactions and their use in analytical chemistry.
Historically, this lab has explored many fundamental aspects of ion-ion reactions, such as the kinetics of consecutive proton transfer reactions, as well as the likelihood of a given reagent to undergo proton transfer or electron transfer. We have also looked at utilizing selective gas-phase ion/ion reactions to yield structural information about polypeptides, by oxidizing disulfide bonds in intermolecularly-linked polypeptide ions.
Recent work has explored the many reaction possibilities that can arise from complex formation. One such area of work has involved charge inversion reactions. Charge inverting a negatively charged lipid can unveil structural features of the acyl chains, which cannot easily be determined in negative mode. Mass Analysis of Macromolecule Analytes via Multiply-charged Ion Attachment (MAMA MIA) is another application where the formation of a complex can lead to structural information. In native mass spectrometry, proteins and protein complexes can preserve some native-like structure in the gas phase, though their individual charge states may be hidden by extensive salt adduction and narrow charge-state spacings. By attaching a protein with a known mass and charge to this analyte, the charge can be reduced while the mass is increased, spreading out the charge states over the mass spectrum, allowing high confidence in mass and charge state assignments.
© 2024, McLuckey Research Group | Division of Analytical Chemistry
Department of Chemistry, Purdue University
560 Oval Drive, West Lafayette, IN 47909