Kenttämaa Labs
Analytical & Physical Organic Chemistry
Research on the development of biofuels
![]()
Conversion of lignocellulosic biomass, including lignin, into biofuels and value-added chemicals requires the degradation of the biomass, which usually generates very complex mixtures. These mixtures cannot be subjected to further refinement without the knowledge of their chemical compositions. Tandem mass spectrometry based on collision-activated dissociation (CAD) is a powerful technique for the characterization of individual compounds in lignin degradation products. However, structural elucidation of unknown lignin-related compounds requires fundamental understanding of how ionized lignin-related compounds fragment upon CAD. Unfortunately, only limited knowledge is available on this topic. Therefore, we have carried out intensive studies on the fragmentation mechanisms of deprotonated lignin model compounds with β-O-4 and other linkages by using deuterium labeling, synthesis of authentic compounds, and quantum chemical calculations. The knowledge obtained from these model compound studies has been used to characterize the structures of the major components in mixtures generated from biomass via the organosolv process. The individual analytes of the organosolv lignin mixtures were separated by using high-performance liquid chromatography and analyzed by using multistage high-resolution tandem mass spectrometry based on CAD (HPLC/HRMSn).
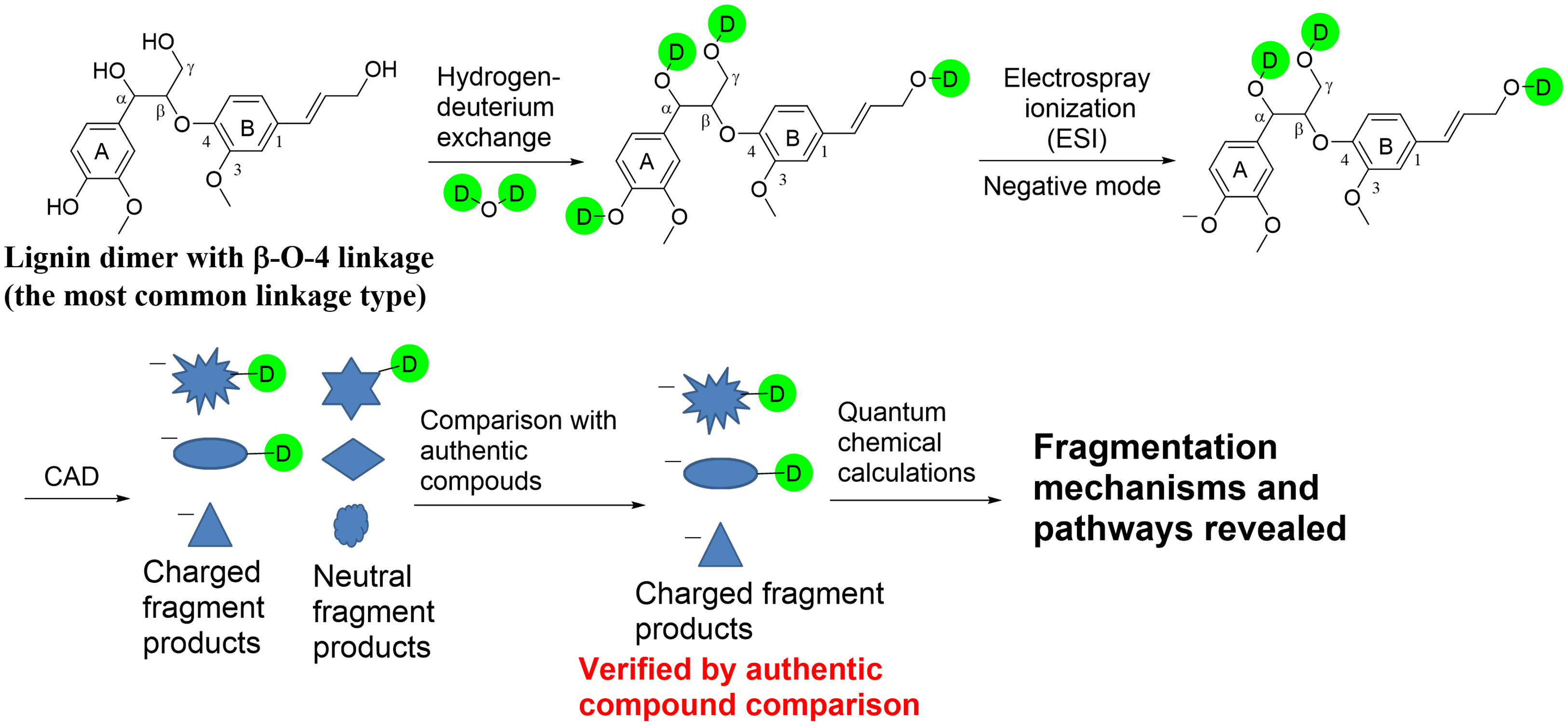
Figure 23. General workflow for some of the studies on the fragmentation mechanisms of deprotonated lignin model compounds.

Figure 24. General workflow for the analysis of complex lignin degradation mixtures by using the HPLC/HRMSn method.
The mechanisms of the three major CAD pathways of deprotonated lignin model compounds with a β-O-4 linkage were identified. The structures of the key fragment ions were determined by comparison of the CAD mass spectra measured for ionized, undeuterated and deuterated analogs and for deprotonated authentic compounds. Some of the proposed reaction mechanisms were examined by studying additional deprotonated synthetic model compounds. Quantum chemical calculations were used to delineate the most likely reaction pathways and reaction mechanisms.
The HPLC/HRMSn method was used to partially separate the unknown analytes in an organosolv poplar lignin mixture and to obtain structural information for many of the unknown analytes. The structures of 62 unknown compounds in the mixture were elucidated. Based on the proposed structures, compounds in the organosolv lignin sample contain β-O-4, 5-5, β-5, and possibly also 4-O-5 linkages.
References:
- Sheng, H.; Tang, W.; Gao, J.; Riedeman, J. S.; Li, G.; Jarrell, T. M.; Hurt, M. R.; Yang, L.; Murria, P.; Ma, X.; Nash, J. J.; Kenttämaa, H. I. (−)ESI/CAD MSn Procedure for Sequencing Lignin Oligomers Based on a Study of Synthetic Model Compounds with β-O-4 and 5-5 Linkages. Anal. Chem. 2017, 89, 13089–13096.
- Sheng, H.; Murria, P.; Degenstein, J. C.; Tang, W.; Riedeman, J. S.; Hurt, M. R.; Dow, A.; Klein, I.; Zhu, H.; Nash, J. J.; Abu‐Omar, M.; Agrawal, R.; Delgass, W. N.; Ribeiro, F. H.; Kenttämaa, H. I. Initial Products and Reaction Mechanisms for Fast Pyrolysis of Synthetic G-Lignin Oligomers with β-O-4 Linkages via On-Line Mass Spectrometry and Quantum Chemical Calculations. ChemistrySelect 2017, 2, 7185–7193.
- Zhu, H.; Max, J. P.; Marcum, C. L.; Luo, H.; Abu-Omar, M. M.; Kenttämaa, H. I. Identification of the Phenol Functionality in Deprotonated Monomeric and Dimeric Lignin Degradation Products via Tandem Mass Spectrometry Based on Ion–Molecule Reactions with Diethylmethoxyborane. J. Am. Soc. Mass Spectrom. 2016, 27, 1813–1823.
- Marcum, C. L.; Jarrell, T. M.; Zhu, H.; Owen, B. C.; Haupert, L. J.; Easton, M.; Hosseinaei, O.; Bozell, J.; Nash, J. J.; Kenttämaa, H. I. A Fundamental Tandem Mass Spectrometry Study of the Collision-Activated Dissociation of Small Deprotonated Molecules Related to Lignin. ChemSusChem 2016, 9, 3513–3526.