Kenttämaa Labs
Analytical & Physical Organic Chemistry
Laser-induced acoustic desorption experiments (LIAD)
![]()
The design, construction and characterization of two different laser-induced acoustic desorption (LIAD) devices is underway for the evaporation of large, thermally labile compounds as neutral molecules into tandem mass spectrometers. The goals include the ability to use tandem mass spectrometry to explore reactions of charged radicals with thermally labile biomolecules, such as polypeptides and oligonucleotides, in a quadrupole ion trap. These biomolecules cannot be introduced into mass spectrometers as neutral compounds by heating as they degrade upon heating. Therefore, special laser-based methods are needed for these studies.
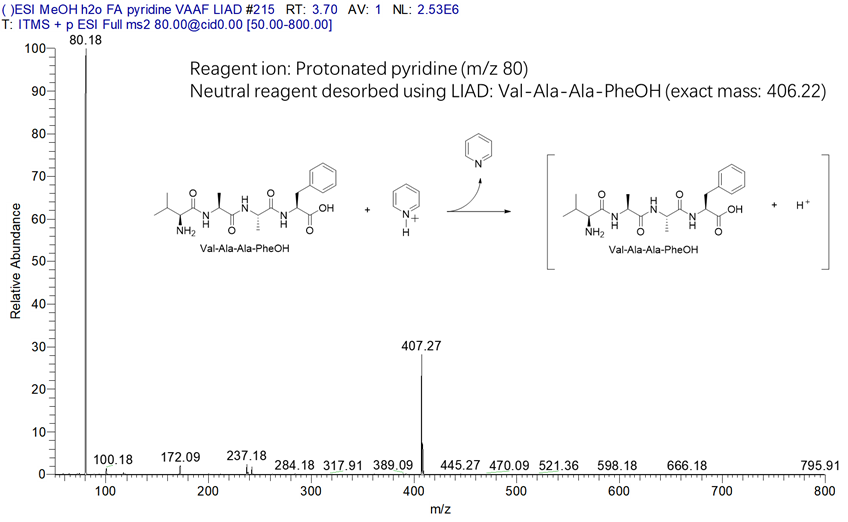
Figure 18. Mass spectrum collected in a LIAD experiment for the reaction of protonated pyridine (m/z 80) with a tetrapeptide (Val-Ala-Ala-PheOH; MW 406 Da) in a linear quadrupole ion trap. The tetrapeptide was desorbed into the linear quadrupole ion trap by using LIAD and protonated by protonated pyridine.
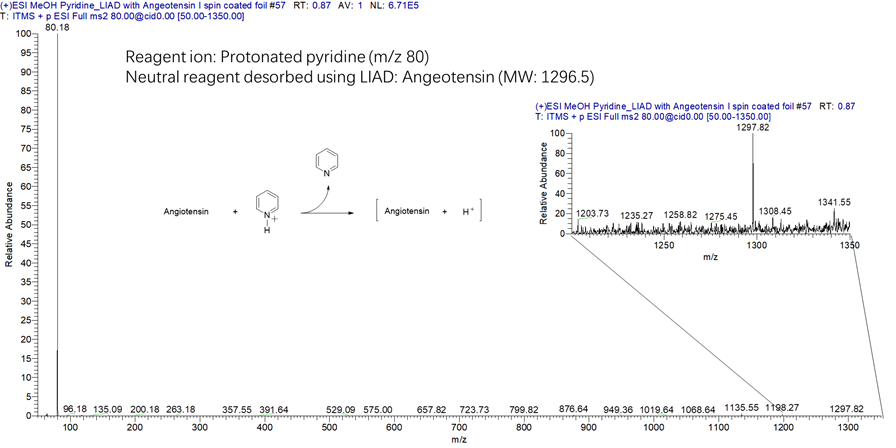
Figure 19. Mass spectrum measured for the reaction of protonated pyridine (m/z 80) with a large peptide (Angiotensin I; MW 1297 Da) desorbed into the ion trap via LIAD. Protonated pyridine protonated the peptide.
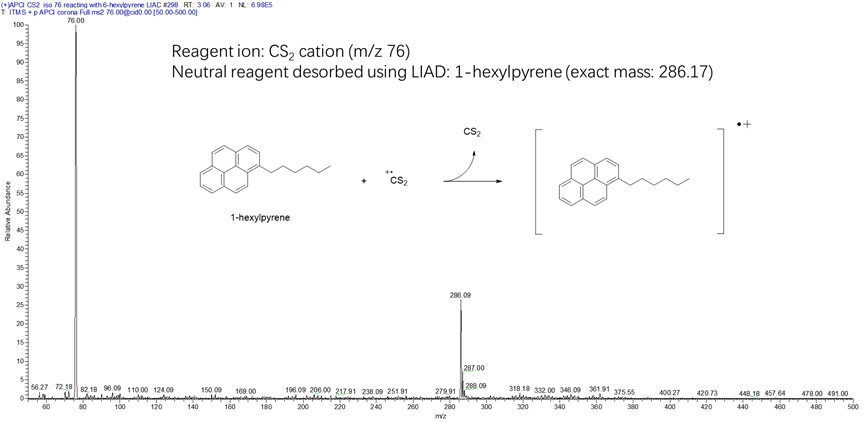
Figure 20. Mass spectrum measured for the reaction of the CS2 radical cation with an asphaltene model compound (1-hexylpyrene; MW 286 Da). The model compound was desorbed into a linear quadrupole ion trap via LIAD and ionized via electron transfer reaction with the CS2 radical cation (m/z 76).
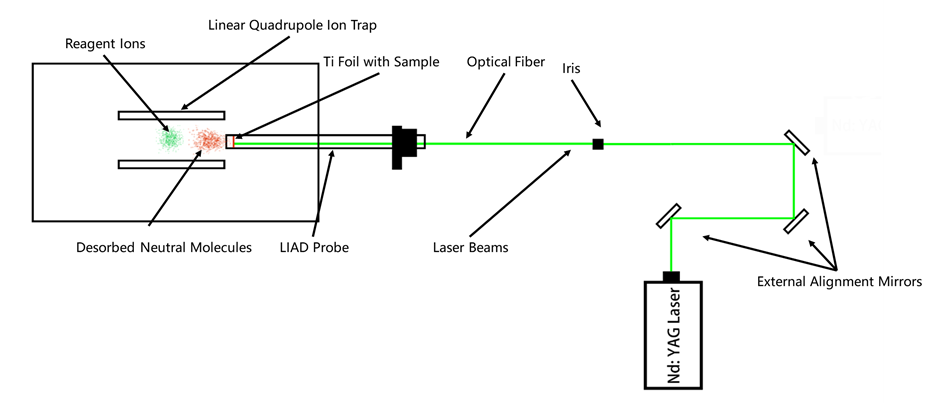
Figure 21. The instrument configuration of the LIAD device used in the above experiments.1
Reference:
- Lei, H.-R.; Zhang, Y.; Kenttämaa, H. I. Design and Characterization of a Dual Laser-Induced Acoustic Desorption Setup with a Linear Quadrupole Ion Trap Mass Spectrometer (manuscript in preparation)