Kenttämaa Labs
Analytical & Physical Organic Chemistry
Polyradicals
We have pioneered the study of the chemical properties of σ-type carbon-centered aromatic bi- and polyradicals. These studies are based on gas-phase ion-molecule reactions occurring in ion trap mass spectrometers. Some of our research objects are listed below:
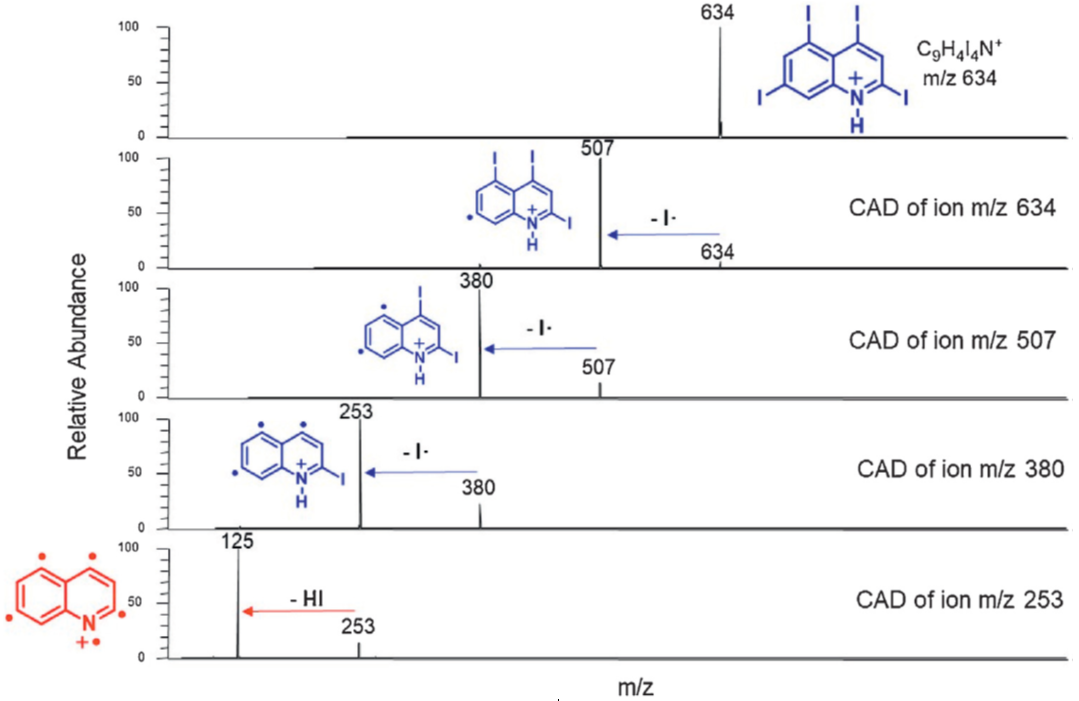
Discovery of novel organic bi- and polyradicals via the synthesis of diverse polyradical precursors, such as the ones shown below. These precursors were subjected to collision-activated dissociation in an ion trap mass spectrometer to cleave off the iodine atoms and thereby generate radical sites at the carbons at which they were bound.
Figure 7. Synthesis of some iodinated precursors for organic biradicals.
Below figure shows an example of the generation of a polyradical in the ion trap mass spectrometer.
References:
- Ma, X.; Feng, E.; Jiang, H.; Boulos, V.; Gao, J.; Nash, J. J.; Kenttämaa, H. I. Protonated Ground-State Singlet meta-Pyridynes React from an Excited Triplet State. J. Org. Chem. 2021, 86, 3249-3260.
- Max, J. P.; Ma, X.; Kotha, R. R.; Ding, D.; Milton, J.; Nash, J. J.; Kenttämaa, H. I. Reactivity of Organic σ,σ,σ,σ,σ-Pentaradicals. Intl. J. Mass Spectrom. 2019, 435, 280-290.
Examination of the reactivity of the above bi- and polyradicals toward different organic reagents in order to identify their reactivity-controlling factors, such as polar effects (reflected by the calculated vertical electron affinities of the radical sites; see definition of vertical electron affinity below), which lower the energies of transition states via ionic resonance structures as shown below:
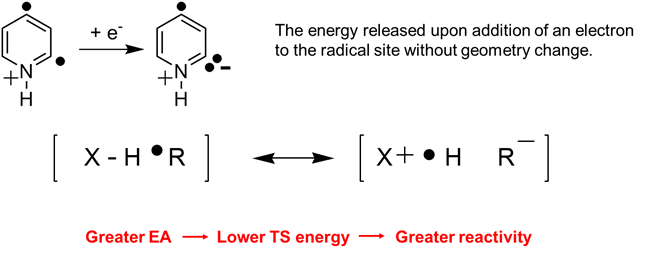
Figure 9. Visualization how polar effects lower the energy of a transition state of a radical reaction.
Reference:
- Heidbrink, J.L.; Ramírez-Arizmendi, L.E.; Thoen, K.K.; Guler, L.; Kenttämaa, H.I. Polar Effects Control Hydrogen-abstraction Reactions of Charged, Substituted Phenyl Radicals. Phys. Chem. A 2001, 105(33), 7875-7884.
Examination of the gas-phase reactivity of bi- and polyradicals toward small biomolecules, including small peptides and oligonucleotides, thus exploring the potential of using polyradicals as the biologically active forms of new drugs, such as anti-cancer agents. Shown below is a mechanism of a reaction of a biradical that leads to the cleavage of an oligonucleotide in the gas phase.
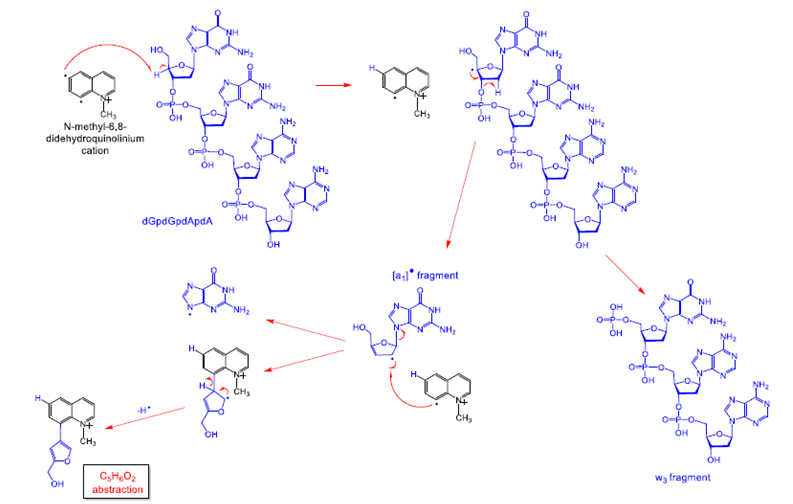
Figure 10. Gas-phase reaction of a charged biradical with a tetranucleotide.
Reference:
- Widjaja, F.; Max, J.P.; Jin, Z.; Nash, J.J.; Kenttämaa, H.I. Gas-phase Reactivity of meta-Benzyne Analogs Toward Small Oligonucleotides of Differing Lengths. J. Am. Soc. Mass Spectrom. 2017, 28(7), 1392-1405.