Kenttämaa Labs
Analytical & Physical Organic Chemistry
Chlorine-containing compounds in waste cooling oils
![]()
To find sustainable energy resources, many energy suppliers have changed their focus from fossil resources to their potentially renewable substitutes, such as waste food, waste plastics, and agricultural residues. Unfortunately, most of the compounds in these materials contain more heteroatoms than the compounds in crude oil. With this in mind, many studies have been performed to identify and quantify potentially harmful sulfur- and nitrogen-containing compounds in these crude materials.1,2 However, some minor components have been ignored although they may play a crucial role in the utility of the final product. Chlorine-containing compounds belong to this group. Indeed, fuels generated, for example, from waste cooking oil usually contain more chlorine compounds than commercial diesel fuel. During the combustion process, chlorine-containing organic compounds generate inorganic hydrogen chloride vapors and chloride salts as well as small chlorine-containing organic compounds, which can harm mechanical parts of combustion equipment, thus decreasing their lifetime.3 Therefore, limiting the amount of chlorine-containing compounds in the fuel generated from daily wastes is important for environmental and economic reasons.
Our group collaborates with NESTE, a Finnish oil company, to develop new methodologies for the identification and quantitation of chlorine containing compounds in waste cooking oil samples by using tandem mass spectrometry. A fractionation method (solid-phase extraction or SPE) was developed to separate the cooking oil sample into five fractions, each containing different types of compounds, that were then analyzed by high-resolution mass spectrometry. Optimal ionization methods were identified for different types of chlorine-containing model compounds before analysis of actual waste cooking oil samples. Collision-activated dissociation experiments were performed on ionized model compounds in order to identify fragmentations that can provide structural information for the unknown chlorine-containing compounds. Reversed-phase high-performance liquid chromatography (HPLC)/negative ion mode electrospray ionization ((-)ESI)/high-resolution mass spectrometry (HRMS) was ultimately utilized for the separation and identification of chlorine-containing compounds in the cooking oil samples and for their quantitation in the polar SPE fraction of waste cooking oil samples.
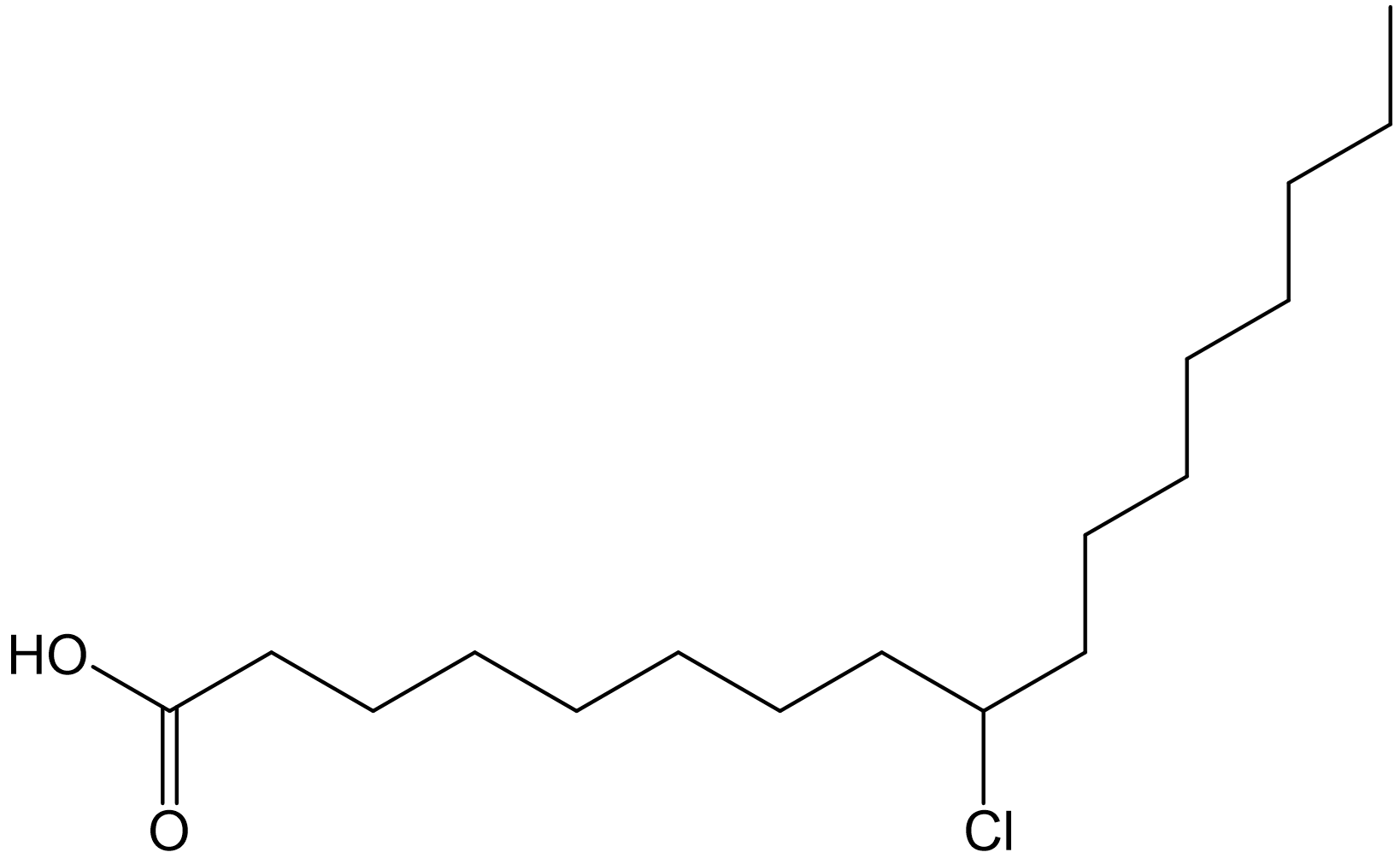
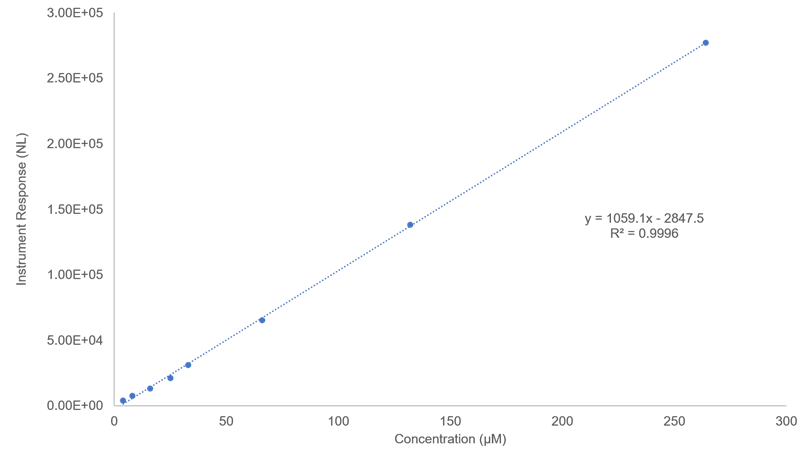
Figure 17. Calibration plot for monochlorinated oleic acid (linear range: 4 µM - 264 µM). Instrument response obtained using reversed-phase HPLC/(-)ESI/MS method; all analytes were dissolved in methanol solvent. Only response for the analyte with 35Cl isotope was recorded.
References:
- He, B. B.; Van Gerpen, J. H.; Thompson, J. C. Sulfur Content in Selected Oils and Fats and Their Corresponding Methyl Esters. Eng. Agric. 2009, 25 (2), 223-226.
- Yu, J.; Maliutina, K.; Tahmasebi, A. A Review on the Production of Nitrogen-containing Compounds from Microalgal Biomass via Pyrolysis. Technol. 2018, 270, 689-701.
- Olek, M.; Baron, J.; Zukowski, W. Thermal Decomposition of Selected Chlorinated Hydrocarbons during Gas Combustion in Fluidized Bed. Cent. J. 2013, 7 (1), 2.