Kenttämaa Labs
Analytical & Physical Organic Chemistry
Carbyne anions
Carbynes, the monovalent carbon radical family comprising CH and its derivatives, represent the least explored and most poorly understood variety of carbon radicals.1 Carbyne anions are even more rare as only one has been reported in the literature.2
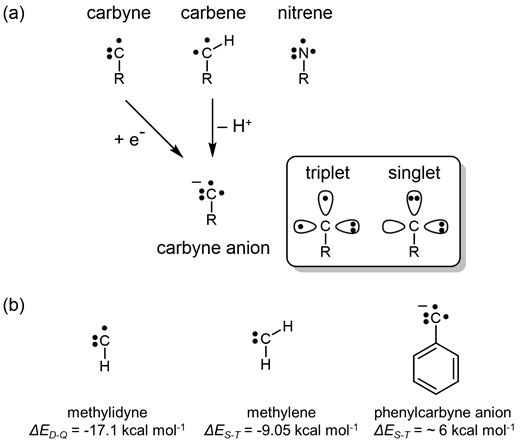
Figure 11. Generic structures for a doublet carbyne, a triplet carbene, a triplet nitrene and a triplet carbyne anion, and the electronic structures of triplet and singlet carbyne anions. (b) The structures of the simplest doublet carbyne, singlet carbene and triplet phenylcarbyne anion and the relevant doublet-quartet and singlet-triplet splittings.1-3
We have generated several carbyne anions by using electrospray ionization in the negative ion mode to ionize tetrazole precursors in a linear quadruple ion trap and then subjecting them to collision-activated dissociation to generate the carbyne anions via elimination of two nitrogen molecules, as shown below. We are in the process of systematically examining their chemical properties in the gas phase. The experimental studies are complemented by multi-configuration SCF calculations.
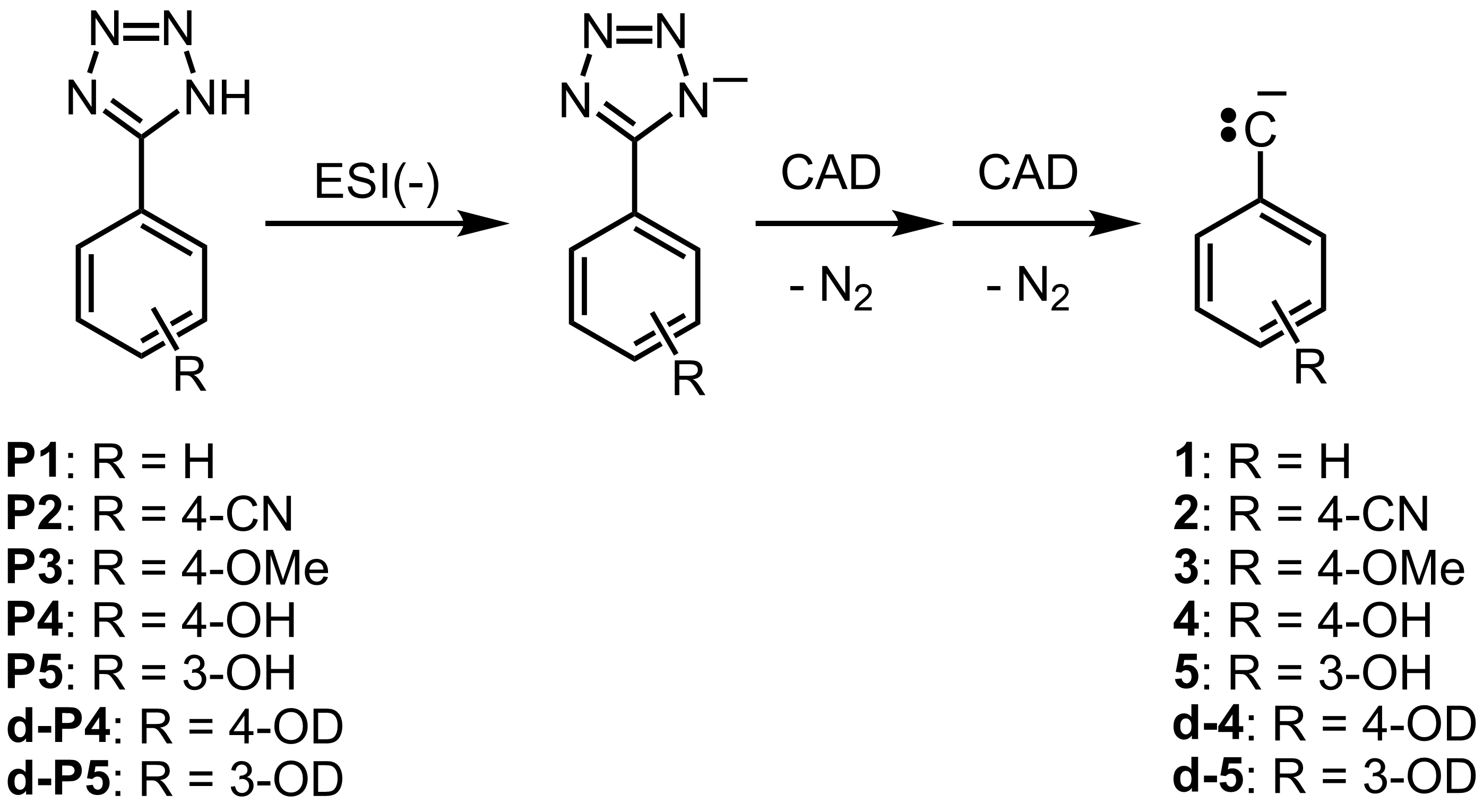
Figure 12. Generation of the phenylcarbyne anions 1 - 5, d-4 and d-5 from the precursors P1 - P5, d-P4 and d-P5, respectively.4
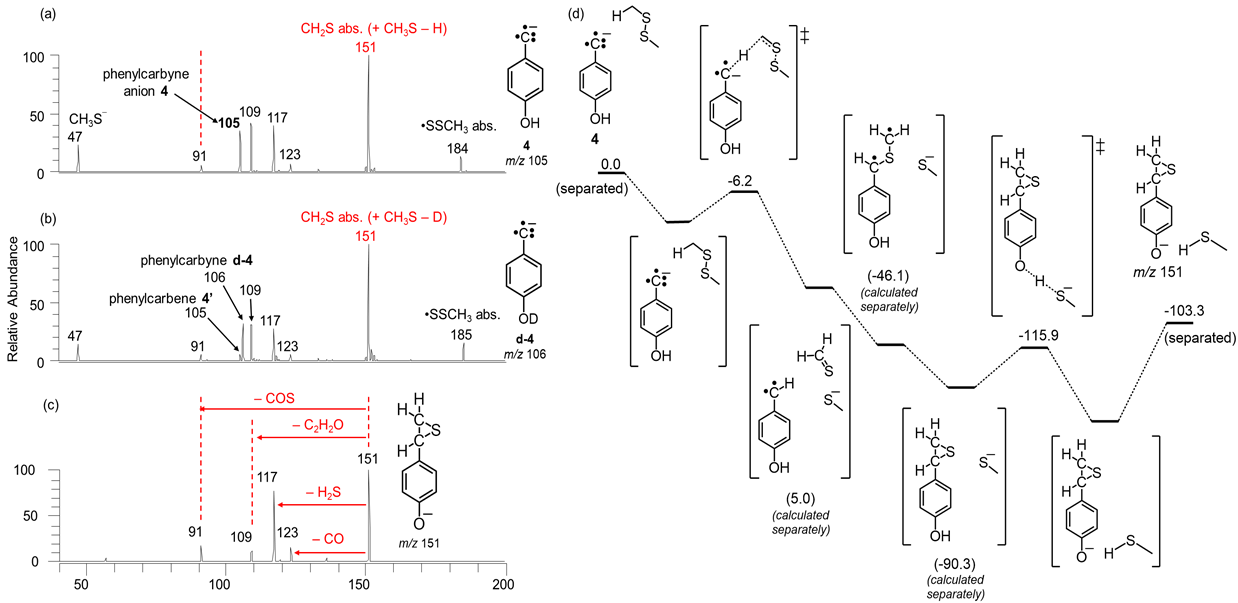
Figure 13. (a) Mass spectrum measured after 100 ms reaction of phenylcarbyne anion 4 with dimethyl disulfide. (b) Mass spectrum measured after 100 ms reaction of phenylcarbyne anion d-4 with dimethyl disulfide. (c) CAD mass spectra of the product ions of m/z 151 isolated after reaction of 4 with dimethyl disulfide (Figure 6a). (d) Potential energy surface (enthalpies in kcal mol-1) calculated at the M06-2X/6-311++G(d,p) level of theory for the generation of CH3S¯ for 4 (as shown in Figure 4 for 1) followed by abstraction of a proton by CH3S¯ to yield the CH2S abstraction product (to generate ions of m/z 151).
References:
- Hou, Z. and Bayes, K.D. Rate Constants for the Reaction of Methyne with Nitric Oxide, Dinitrogen, Nitrous Oxide, Carbon Monoxide, Carbon Dioxide and Water. Phys. Chem. 1993, 97, 1896-1900.
- Seburg, R. A., Hill, B. T., Jesinger, R. A. and Squires, R. R. The Phenylcarbyne Anion. Am. Chem. Soc. 1999, 121, 6310-6311.
- Cramer, C.J., Dulles, F.J. and Falvey, D.E. Ab Initio Characterization of Phenylnitrenium and Phenylcarbene: Remarkably Different Properties for Isoelectronic species. Am. Chem. Soc. 1994, 116, 9787-9788.
- Liu, W., Guo, Y., Han, C. and Huang, X. Characteristic Fragmentation Behavior of 5-[1-Aryl-1H-pyrrol-2-yl]-1H-tetrazole by Electrospray Ionization Tandem Mass Spectrometry. Life Sci. J. 2008, 5, 25-29.