
Research
ABC Transporters: Roles in Multidrug Resistance and Bioavailability
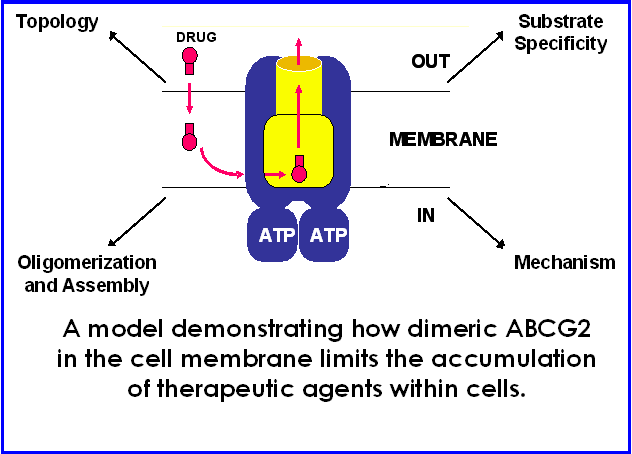
One of our research interests is in the field of multidrug resistance in human cancer. Although numerous cancers can be successfully treated with ablative surgery, radiotherapy or chemotherapy, many cancers are intrinsically resistant to anti-cancer drugs or become resistant through the course of treatment. This broad-based cellular resistance to anti-cancer drugs results, in large part, from expression of a 170 kDa multidrug transporter or P-glycoprotein, encoded by the multidrug resistance MDR1 gene in humans. Many different human cancers express the MDR1 gene at levels sufficient to confer multidrug resistance and it can be estimated that approximately 50% of human cancers will express the gene at some time during therapy.
Therefore, it has become apparent that multidrug resistance in a clinical setting is an obstacle that must be overcome to treat cancers effectively. It is of obvious importance to fully understand how the transporter functions in order to combat this phenomenon clinically. Taking biochemical, molecular genetic, and cell biological approaches using both mammalian and microbial cell systems, the majority of our efforts focus on the elucidation of the mechanism of action of human P-glycoprotein and a related drug transporter ABCG2 that is involved in mitoxantrone resistance. Ultimately, a complete understanding of these proteins could lead to the development of new inhibitory agents that could greatly facilitate the treatment of a large number of human cancers.
These drug transporters are members of a large superfamily of membrane transporters called the ATP-Binding Cassette (ABC) family. Through work with P-glycoprotein and ABCG2, our interest in the field of transporters and their relation to cellular function and human disease has developed. In the future, we are also interested in identifying and studying other ABC transporters possibly linked to human diseases. We hope to gain further understanding of the basic mechanism of action of ABC transporters to elucidate their cellular functions both normally and in potential disease states. Human ABCG2 is 655 amino acids in length with six predicted transmembrane domains and one soluble nucleotide binding domain (NBD) and is believed to form a functional dimer.
Mammalian -CaaX Protein Processing
We are also interested in the post-translational modifications of eukaryotic proteins, many of which are involved in signal transduction, including the small G-proteins. These proteins are initially synthesized with the C-terminal sequence –Cys-Xaa-Xaa-Xaa and undergo a series of three modifications that include isoprenylation of the cysteine residue, proteolysis of the three terminal residues and α-carboxyl methylesterification of the cysteine residue. These modifications are thought to target otherwise soluble proteins to an intracellular membrane where they carry out their cellular functions. The Ras protein is known to undergo these modifications. When activated ras does not reaching the membrane, it is no longer capable of transforming cells. Therefore, therapeutically, inhibitors of C-terminal isoprenylcysteine methylation may prove to be useful pharmacological anti-cancer agents. Presently, we are interested in understanding the role of the methylation reaction in protein function more fully and in gaining a more complete biochemical understanding of the enzyme responsible for catalyzing this methylation reaction in both yeast and mammalian cells.
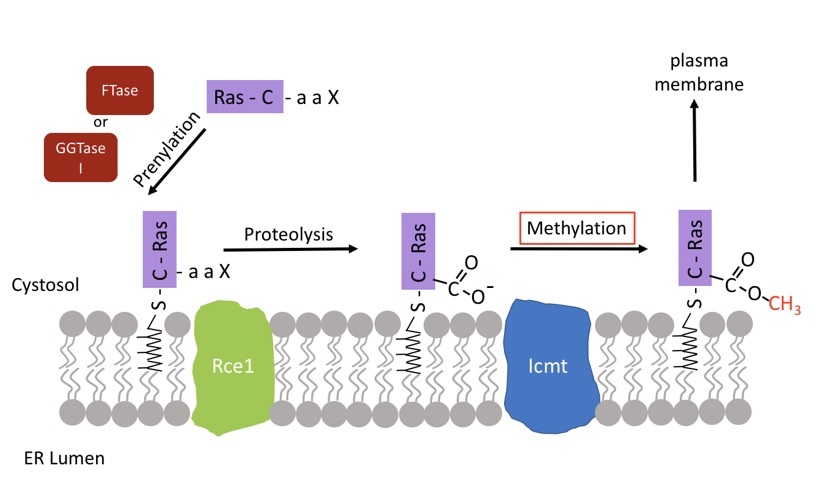
- Normal human Ras CaaX protein processing.
- We study both the Rce1 and Icmt membrane-associated enzymes.
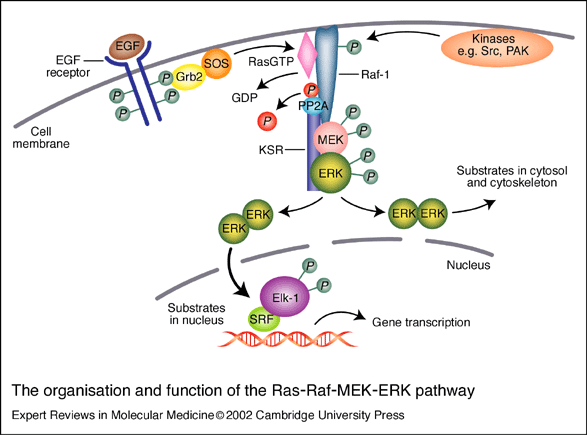
- Mutations in Ras are found in 30% of all human cancers, including 90% of pancreatic carcinomas.
Bergo et al. JCI (2004)113:539
Icmt Inhibition Results in K-Ras Mislocalization
Development of Icmt Inhibitors
HYPOTHESIS: Disruption of the Ras maturation pathway can be accomplished by small molecule inhibition of Icmt.
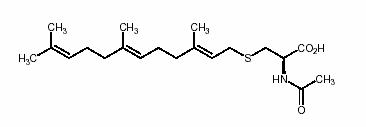
- Normal human Ras CaaX protein processing.
- We study both the Rce1 and Icmt membrane-associated enzymes.
- Modify the acyl & isoprene moieties to probe prenyl binding and active sites and develop inhibitors.
- Inhibitors with low Km and low Vmax most desirable