Structure and function, chemical biology and therapeutic targeting of protein tyrosine phosphatases
As a major posttranslational modification mechanism, protein tyrosine phosphorylation regulates a variety of cellular processes including growth, differentiation, migration, and survival. Proper level of protein tyrosine phosphorylation, which is dynamically maintained by protein
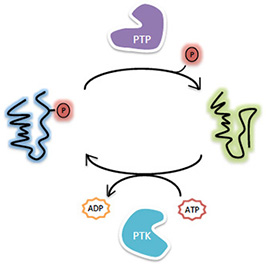 tyrosine kinases (PTKs) and protein tyrosine phosphatases (PTPs), is essential for normal cellular homeostasis. Perturbation to the balance between PTK and PTP activity induces tyrosine kinases (PTKs) and protein tyrosine phosphatases (PTPs), is essential for normal cellular homeostasis. Perturbation to the balance between PTK and PTP activity induces 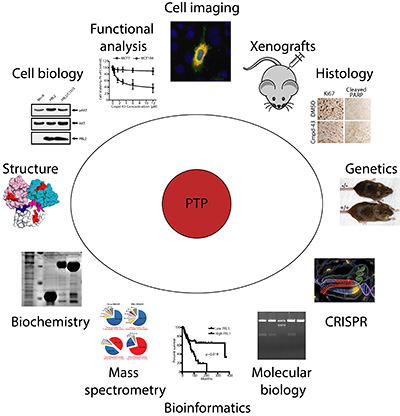 either excessive or diminished substrate phosphorylation, which is associated with a wide range of pathological conditions. Consequently, the ability to selectively modulate signaling pathways mediated by protein tyrosine phosphorylation holds enormous potential for therapeutic intervention, as evidenced by the development of PTK inhibitors for various clinical indications. either excessive or diminished substrate phosphorylation, which is associated with a wide range of pathological conditions. Consequently, the ability to selectively modulate signaling pathways mediated by protein tyrosine phosphorylation holds enormous potential for therapeutic intervention, as evidenced by the development of PTK inhibitors for various clinical indications.
Given the reversible nature of protein tyrosine phosphorylation, there is also potential to manipulate tyrosine phosphorylation mediated signaling pathways at the level of PTPs. The PTPs, encoded by more than 100 genes in humans, constitute a large family of enzymes that parallel PTKs in their structural diversity and complexity. Like PTKs, deregulation of PTP activity has been linked to diabetes/obesity, cancer, and autoimmune dysfunctions, and therefore PTPs are emerging as exciting new drug targets. Despite the increasing interest in this important enzyme family, the function of most PTPs is still not well understood and the PTPs remain a largely under-exploited resource for therapeutic intervention. Among the contributing factors to the failure of targeting PTPs for drug discovery is the lack of detailed understanding of how dysregulation of PTP activity cause human diseases. In addition, the PTPs are exceptionally challenging targets for the development of potent and selective small molecule therapeutics due to the highly conserved and positively charged active sites.
 Research in our laboratory deals with structure and function of PTPs, PTP-mediated cellular signaling mechanisms, roles of PTPs in normal physiology and diseases, chemical biology and drug discovery targeting the PTPs. Major effort is devoted to the development of novel biochemical, chemical biological,
structural and genetic approaches to elucidate the roles of PTPs in normal physiology and in diseases. Specifically, using physiological substrates (i.e., phosphoproteins), we are investigating the molecular basis for PTP catalysis and substrate recognition. Understanding the molecular basis for tyrosine dephosphorylation by PTPs will open doors to new experimental approaches Research in our laboratory deals with structure and function of PTPs, PTP-mediated cellular signaling mechanisms, roles of PTPs in normal physiology and diseases, chemical biology and drug discovery targeting the PTPs. Major effort is devoted to the development of novel biochemical, chemical biological,
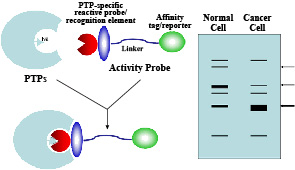
structural and genetic approaches to elucidate the roles of PTPs in normal physiology and in diseases. Specifically, using physiological substrates (i.e., phosphoproteins), we are investigating the molecular basis for PTP catalysis and substrate recognition. Understanding the molecular basis for tyrosine dephosphorylation by PTPs will open doors to new experimental approaches  (such as the creation of PTPs with altered catalytic and regulatory properties and the design and development of specific PTP inhibitors) that will elucidate mechanisms by which these enzymes control cell functions. We are employing high affinity PTP substrate-trapping mutants in combination with mass spectrometry for rapid isolation, identification, and characterization of physiological PTP substrates. Identification and characterization of cellular PTP substrates will help elucidate the function of individual PTPs as well as assignment of PTPs to specific signaling pathways. We are developing activity-based probes to analyze globally profile PTP activity in vivo. The ability to profile the entire PTP family on the basis of changes in their activity should greatly accelerate both the assignment of PTP function and the identification of potential therapeutic targets. We are also generating novel gene knockout and transgenic mouse models to define the roles of PTPs in development, metabolism, and tumorigenesis, both at the cellular and organismal levels. We are also using these models to identify and validate novel PTPs as drug targets. (such as the creation of PTPs with altered catalytic and regulatory properties and the design and development of specific PTP inhibitors) that will elucidate mechanisms by which these enzymes control cell functions. We are employing high affinity PTP substrate-trapping mutants in combination with mass spectrometry for rapid isolation, identification, and characterization of physiological PTP substrates. Identification and characterization of cellular PTP substrates will help elucidate the function of individual PTPs as well as assignment of PTPs to specific signaling pathways. We are developing activity-based probes to analyze globally profile PTP activity in vivo. The ability to profile the entire PTP family on the basis of changes in their activity should greatly accelerate both the assignment of PTP function and the identification of potential therapeutic targets. We are also generating novel gene knockout and transgenic mouse models to define the roles of PTPs in development, metabolism, and tumorigenesis, both at the cellular and organismal levels. We are also using these models to identify and validate novel PTPs as drug targets.
Concurrent effort is directed to accelerate therapeutic targeting of the PTPs, we have established a unique academic chemical genomic program encompassing high-throughput screening, structure-based design, and medicinal chemistry to develop small molecule PTP probes for functional interrogation, target identification/validation, and therapeutic development. To this end, we have pioneered a novel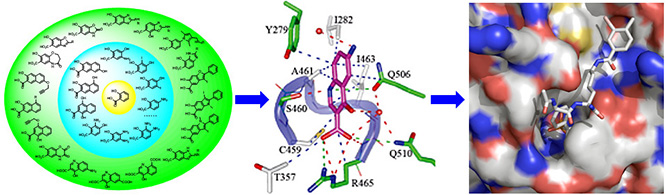 paradigm for the acquisition of potent and selective PTP inhibitors by targeting both the PTP active site and unique pockets in the vicinity of the active site. We are also employing a novel drug repurposing strategy to explore the existing drug space for previously unknown PTP inhibitory scaffolds that can be further optimized into potent and selective PTP inhibitors with improved drug-like properties. We have developed a number of nonhydrolyzable pTyr pharmacophores that are sufficiently polar to bind the PTP active site, yet remain capable of efficiently crossing cell membranes, offering PTP inhibitors with both high potency and excellent in vivo efficacy in animal models of oncology, diabetes/obesity, autoimmunity, and tuberculosis. Current efforts aim to advance our lead generation paradigms and to create a ‘PTP-based drug discovery platform’ that will ultimately impact broadly the portfolio of tomorrow. paradigm for the acquisition of potent and selective PTP inhibitors by targeting both the PTP active site and unique pockets in the vicinity of the active site. We are also employing a novel drug repurposing strategy to explore the existing drug space for previously unknown PTP inhibitory scaffolds that can be further optimized into potent and selective PTP inhibitors with improved drug-like properties. We have developed a number of nonhydrolyzable pTyr pharmacophores that are sufficiently polar to bind the PTP active site, yet remain capable of efficiently crossing cell membranes, offering PTP inhibitors with both high potency and excellent in vivo efficacy in animal models of oncology, diabetes/obesity, autoimmunity, and tuberculosis. Current efforts aim to advance our lead generation paradigms and to create a ‘PTP-based drug discovery platform’ that will ultimately impact broadly the portfolio of tomorrow.
|