Laboratory Experiments
Laboratory experiments are essential for obtaining fundamental understanding of particle chemistry and their possible effects on the environment. In partnership with other research groups, we design and conduct physical chemistry experiments to probe the composition and atmospheric aging of model biogenic and anthropogenic organic aerosol in laboratory settings mimicking atmospheric conditions of relative humidity, temperature, pressure, reaction time, trace reactive gas concentrations, etc. Recent examples of our Laboratory Experiments are listed below.
Molecular Investigation of the Multi-phase Photochemistry of Fe(III)–Citrate in Aqueous Solution
Photocatalytic reactions of Fe(III)–carboxylate complexes in nature play an important role in oxidation of dissolved organic constituents and control the bioavailability of iron for development of microbial life. This work presents an experimental study on the molecular photochemistry of an Fe–citrate model system, describing in detail the sequence of its photochemical reactions in the aqueous solution. We characterize water soluble organic and Fe–organic components of the reaction system and reveal the formation of insoluble colloidal products containing carbon in the reduced oxidation state. The analytical and physical chemistry methods developed in our work are now extended to study the photochemistry of other Fe–carboxylate systems.
(Project supported by NSF grant No. AGS-2039985)
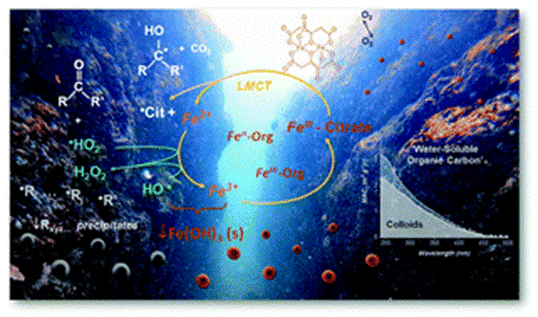
Image Caption: A schematic artwork illustrating Reaction sequence of the photo-catalytic redox cycle of the Fe(III)–citrate complex in an aquatic environment.
Relevant publication
C.P. West, J. Ryan, A.C. Morales, M.M. Misovich, A.P.S. Hettiyadura, F. Rivera-Adorno, J.M. Tomlin, A. Darmody, B.N. Linn, P. Lin, A. Laskin* Molecular Investigation of the Multi-Phase Photochemistry of Fe(III)-Citrate in Aqueous Solution. Environmental Science: Processes & Impacts, (2022). Published online, doi: 10.1039/D1EM00503K
Studies of Secondary Brown Carbon Produced from the Photooxidation of Naphthalene
We investigate the chemical composition of organic light-absorbing components, termed brown carbon (BrC) chromophores, formed in a proxy of anthropogenic secondary organic aerosol generated from the photooxidation of naphthalene (naph-SOA) in the absence and presence of NOx. We provide molecular-level insights into the chemical composition and optical properties of individual naph-SOA components and investigate their BrC relevance. Our study revealed formation of strongly absorbing nitro-aromatic chromophores under high-NOx conditions and described their degradation during atmospheric aging.
(Project supported by NSF grant No. AGS-2039985)
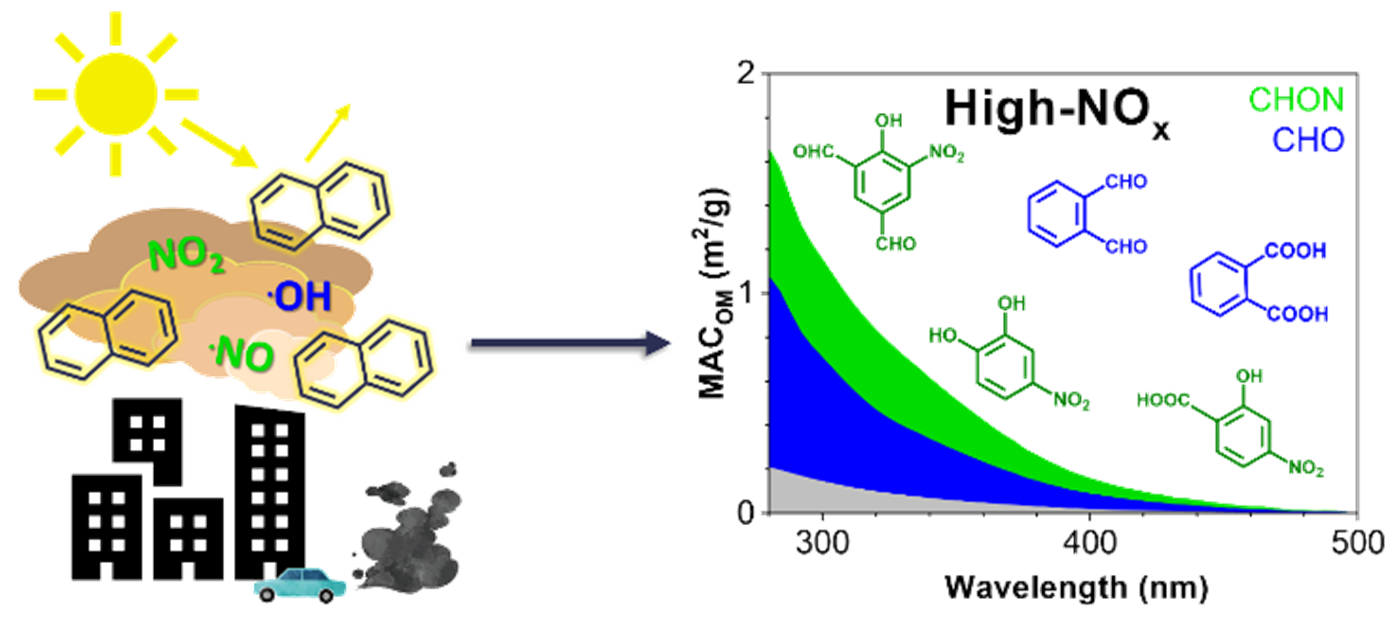
Image Caption: A schematic artwork illustrating contributions of secondary formed nitrophenol BrC chromophores to the light absorbing properties of naph-SOA quantified as mass absorption coefficient (MAC) presented in the insert plot.
Relevant publication
Siemens, A. Morales, Q. He, C. Li, A.P.S. Hettiyadura, Y. Rudich, A. Laskin. Molecular Analysis of Brown Carbon Components of Secondary Organic Aerosol Produced from Photooxidation of Naphthalene. Environmental Science and Technology, 56 (6), 3340-3353, (2022). doi: 10.1021/acs.est.1c03135
Formation of Brown Carbon Products from Aqueous-Phase Photo-Oxidation of Organic Mixtures
We study the photolytic reactions of lab proxy systems containing compounds found in biomass burning emissions. Our study provides novel mechanistic insights into the multitude of reactions that occur during photochemical processing of aqSOA produced from biomass burning emissions. The results indicate that three generations of products formed at different stages of the experiment: monomeric products, dimeric products, and less polar aromatic products similar to those formed during oxygen-deprived pyrolytic processes. The monomeric and dimeric products result from oxidation reactions initiated by triplet excited carbon (3C*), while the less polar aromatic products form as a result of radical recombination. We show that longer irradiation times lead to CO and CO2 degassing and the formation of pyrolytic-like species in the oxygen-deficient aqueous phase. These species enhance BrC optical properties and they are photorecalcitrant.
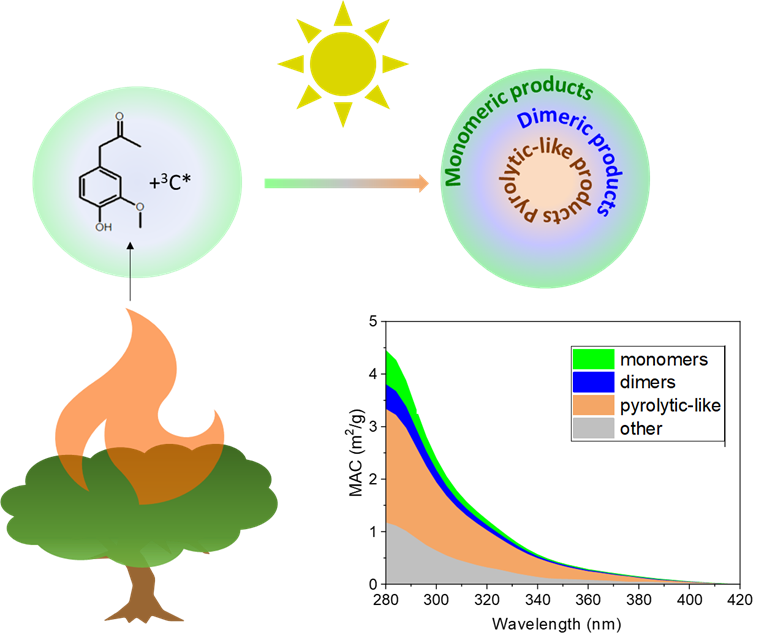
Image Caption: A schematic artwork illustrating contributions of monomeric products, dimeric products, and less polar aromatic products formed by aqueous-phase photochemistry to the BrC light absorbing properties quantified as mass absorption coefficient (MAC) presented in the insert plot.
Relevant publication
M. Misovich, A. Hettiyadura, W. Jiang, Q. Zhang, A. Laskin. “A Molecular Level Study of the Photo Oxidation of Aqueous Phase Guaiacyl Acetone in the Presence of 3C*: Formation of Brown Carbon Products” ACS Earth and Space Chemistry, 5, 1983–1996, (2021). doi: 10.1021/acsearthspacechem.1c00103
