Synthetic Glycans & Glycomimetics
We have a long-standing interest in the biological recognition of cell-surface carbohydrates (glycans) and their roles in mediating cellular behavior. Organic synthesis is used to explore structure-driven hypotheses, based on variations in relative stereochemistry and local charge densities. Synthetic glycans are presented in multivalent format, and screened for high binding avidity against proteins and live cells.
These efforts are complemented by the development of synthetic methods for generating pyranosides with specific sulfate patterns (sulfoforms) or unnatural C5 substituents. Sulfoforms are produced from orthogonally protected intermediates, aided by solid-phase synthesis or extraction; Pyranosides with unnatural C5-substituents are produced from 4-deoxypentenosides (4-DPs), a class of unsaturated pyranosides with similar reactivities as glycals. We have used 4-DPs to generate several types of bioactive L-sugars, including neosamine (in aminoglycoside antibiotics) and L-iduronic acid (a major component of heparan sulfate).
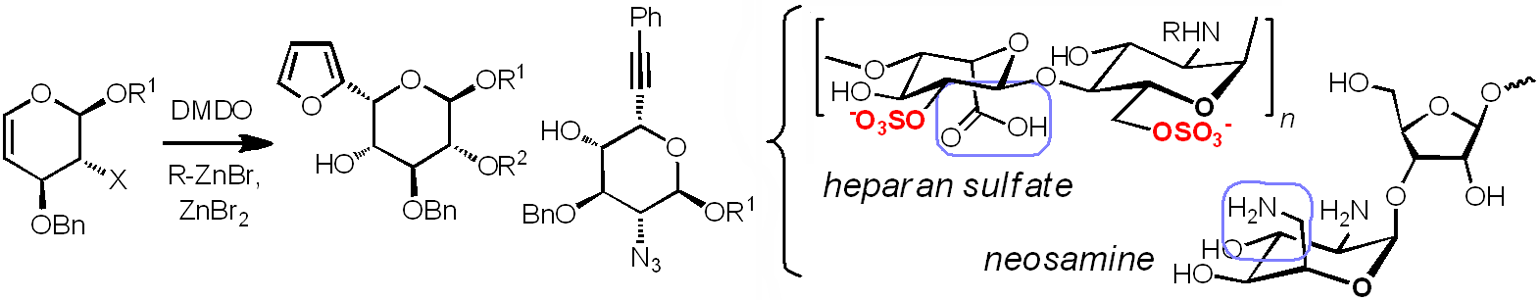
4-Deoxypentenosides (4-DPs) as synthetic intermediates for glycans containing L-sugars.
Several interesting chemistries have emerged in the course of these studies. For example, we have observed that the epoxidation of 4-DPs (as well as many glycals) by DMDO proceeds with high levels of facioselectivity, and is directed by a collective stereoelectronic effect. We have also developed beta-selective glycosylations based on the in situ formation of dithiocarbamates, a versatile functional group with outstanding utility for organic synthesis and surface conjugation.
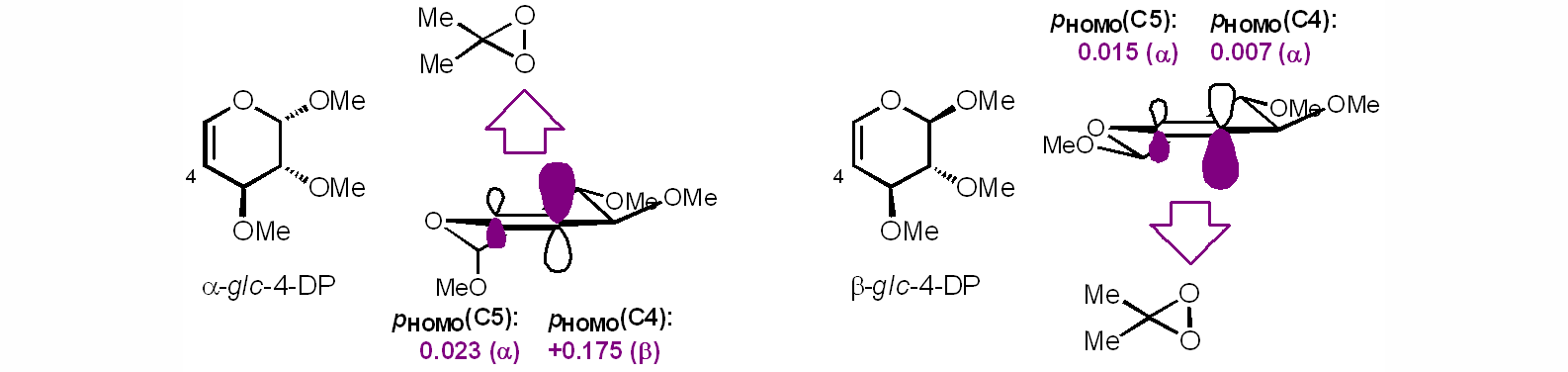
Stereoelectronic effects in the facioselective oxidation of 4-DPs.