Nanoparticles for Imaging and Nanomedicine
Nanoparticles can be designed as multifunctional agents that enhance biomedical imaging, and also for the delivery and release of therapeutic payloads. Rational control over size, shape, and surface properties are critical for the development of nanosized agents with tunable chemical or physical properties. Nanoparticles made in our labs include gold nanorods, nanostars, and hybrid gold/iron-oxide nanoparticles. These nanoparticles can have strong resonances at NIR wavelengths for deeper light penetration into tissues, or respond to magnetic field gradients to actuate physical or biological signals.
Plasmon-resonant gold nanorods (GNRs) and nanostars are NIR-active, polarization-sensitive particles that can be used as contrast agents for various optical imaging modalities. These nanoparticles are also photothermally active, and can be targeted to tumor cells for drug delivery or direct thermolysis. To assess their potential as nanomedicines, we have examined GNRs with various coatings in combination therapies using tumor mouse models, preclinical/nanotoxicology studies, and as siRNA carriers with photothermal release.
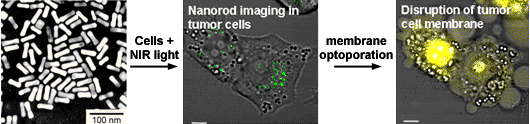
Two-photon excited luminescence from Au nanorods (green) in tumor cells, before and after laser-induced photothermolysis, followed by ethidium bromide staining (yellow).
The ability to detect nanoparticles in complex media like biological tissue is further enhanced by combining plasmon resonance with magnetomotive activity, to support dynamic modes of optical contrast. In the example below, gold nanostars with magnetic cores can produce periodic modulations in polarized NIR scattering in response to magnetic field gradients. Image demodulation by FFT can simultaneously enhance signal quality and reduce background noise, improving contrast by orders of magnitude relative to time-domain images.
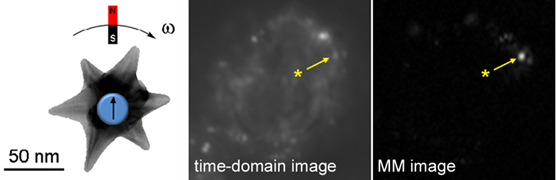
Dynamic (magnetomotive) optical contrast produced by Au nanostars with magnetic cores, shortly after cell uptake by macrophage.
We are also investigating the role of serum proteins on nanomedicine delivery and uptake, guided by proteomic analysis. Nanoparticles in biological media are coated by a corona of proteins that constitute their "biological identity," which has a strong influence on circulation time, biodistribution, and cell uptake. As nanoparticles exit the bloodstream and enter into tissues, the corona may undergo exchange with proteins released by resident cells. By exposing nanoparticles to serum modified by cell cultures, we can study the dynamics of protein corona exchange as a function of its microenvironment, and its impact on nanoparticle uptake by different cell types.