Blocking Efflux Transporters
Chmielewski Group research in drug discovery centers on the identification of biologically active agents through either a rational design approach or from compound library screening and optimization. Current targets in this area include:
Inhibiting Multidrug Resistance Transporters - from the Blood-Brain-Barrier to Malaria
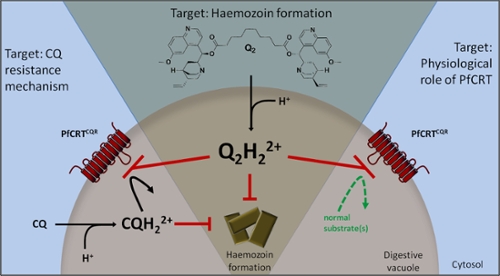
Multidrug resistance transporters consist of families of integral membrane proteins that have recently emerged as major players in limiting the accumulation of therapeutics into specific organs, cells and sub-cellular organelles involved in disease states, such as Alzheimer’s disease, bacterial infection, cancer, epilepsy, HIV, malaria, and schizophrenia, to name a few. For instance, the transporter P-glycoprotein is known to significantly limit the brain penetration of the anti-cancer compound taxol and the anti-HIV agent saquinavir, limiting the ability to treat brain tumors and allowing reservoirs of HIV to persist in the brain. Likewise, the chloroquine resistance transporter of P. falciparum (PfCRT) limits the accumulation of a range of anti-malarial agents into the acidic vacuole of the parasite – the site of therapeutic action – leading to drug resistant forms of malaria. These are just two examples of a range of transporters that have an impact on a significant number of therapies and diseases.
We have pioneered a highly innovative and general strategy to both block the efflux function of transporters and deliver the required therapy to the disease site. To accomplish this goal, we have developed a novel class of agents - dimeric prodrugs of the therapeutic agents themselves. The long term objective of our work is to provide a powerful therapeutic means to overcome drug resistance across a wide range of disease states by targeting a common drug resistance pathway – efflux transporters.
Eradicating HIV Reservoirs in the Brain
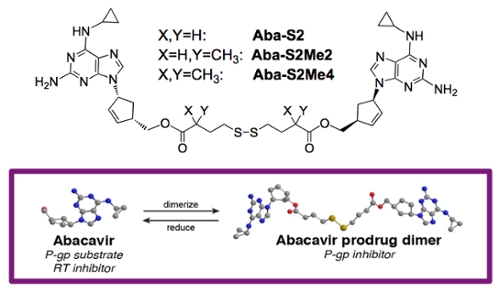
Despite positive developments with the use of combination antiretroviral therapy, a major impediment to limiting the neurocognitive effects of HIV and eradicating brain reservoirs is penetration of these therapies across the blood-brain-barrier. The focus of our work, therefore, is to develop tools to significantly improve the penetration of antiretroviral agents to sites of HIV reservoirs with an emphasis on the CNS. We have designed Trojan horse inhibitors of efflux proteins at the blood-brain barrier composed of dimeric antiviral agents with a traceless tether. These agents play a dual role in inhibiting drug efflux from the brain and regenerating therapy in the reducing environment of brain cells to eradicate HIV.
Eradicating Drug Resistant Malaria
There is a desperate need for new antimalarial drugs due to the spread of drug-resistant strains of P. falciparum. In our Gates Foundation Grand Challenges Explorations funded research, we have successfully developed a highly innovative class of malaria therapies that both block a major drug resistance pathway in P. falciparum and have intrinsic antimalarial activity. In our strategy, we directly attack the mechanism of quinoline-based drug resistance, the P. falciparum transporter PfCRT, with dimers of the resistant therapies themselves. We have developed dimeric antimalarial agents that are the most potent PfCRT inhibitors described to date, and which also display intrinsic antimalarial activity in vitro and in an in vivo animal model. Overall, our work has an immense potential to shift current therapy paradigms away from evasion of drug resistance, to a direct assault on the mechanism of resistance. Our long-term goal is to deliver an inexpensive therapy, based on our platform technology, to eliminate PfCRT-mediated drug resistance in malaria.