Welcome to the Shah Laboratory
Drug Discovery in Cancer and Alzheimer's Disease Using Chemical Biology
Aurora A Kinase and Cancer
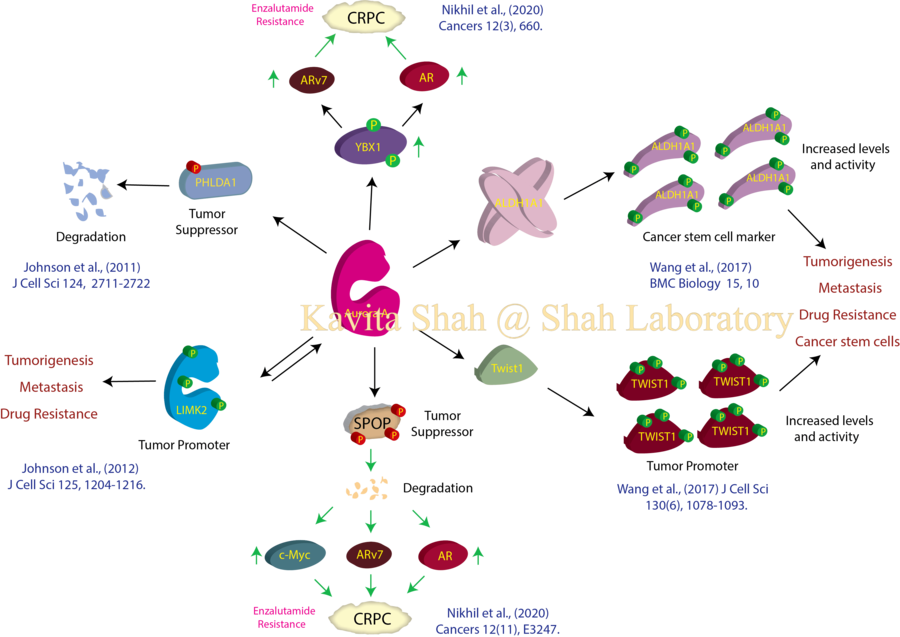
Aurora A kinase (AURKA) is a serine/threonine kinase, which is indispensable for mitosis in normal cells. Aurora A gene is amplified in many types of cancers including breast, prostate, ovarian, colorectal, gastric, pancreatic, hepatocellular, gliomas, nonendometriod, hematological malignancies and aggressive non-Hodkin’s lymphoma. In animal models, Aurora A overexpression induces tumor formation and drug resistance, and its inhibition significantly reduces tumor multiplicity and size. Data such as these have resulted in ongoing clinical trials of more than a dozen Aurora A inhibitors. Despite these encouraging findings, Aurora A inhibition in Phase II clinical trials has been associated with several adverse side effects such as reversible neutropenia, mucositis and somnolence, suggesting that collateral inhibition of Aurora A in rapidly proliferating normal tissues is responsible for the undesirable side effects. These findings indicate that selective targeting of oncogenic targets of Aurora A in cancer may be a superior option for developing effective drugs and combating collateral toxicity. To this end, we have identified several cancer-specific targets of Aurora A in different cancers using a modified chemical genetic approach.
For example- we identified LIMK2 as a direct target of Aurora A in many different cancer types. This was in contrast with PHLDA1, which was identified as Aurora A substrate specifically in ER+ breast cancer (Johnson et al., 2011). Our data demonstrated that Aurora A regulates LIMK2 kinase activity, subcellular localization and protein levels by direct phosphorylation at S283, T494 and T505. In response, LIMK2 also positively regulates the level of Aurora A, thereby engaging in a positive-feedback loop, promoting Aurora-A-mediated oncogenic pathways.
Most importantly, knock-down of LIMK2 in Aurora A-overexpressing highly malignant cancer cells completely abrogates tumorigenesis in nude mice, thereby highlighting LIMK2 as a potential clinical target in highly aggressive cancers. Notably, unlike Aurora A, LIMK2 null mice are viable, signifying that LIMK2 inhibition should cause minimal collateral toxicity in patients, thereby improving overall quality of life (Johnson et al., 2012).