Welcome to the Shah Laboratory
Drug Discovery in Cancer and Alzheimer's Disease Using Chemical Biology
LIMK2 as a Highly Effective Cancer Target
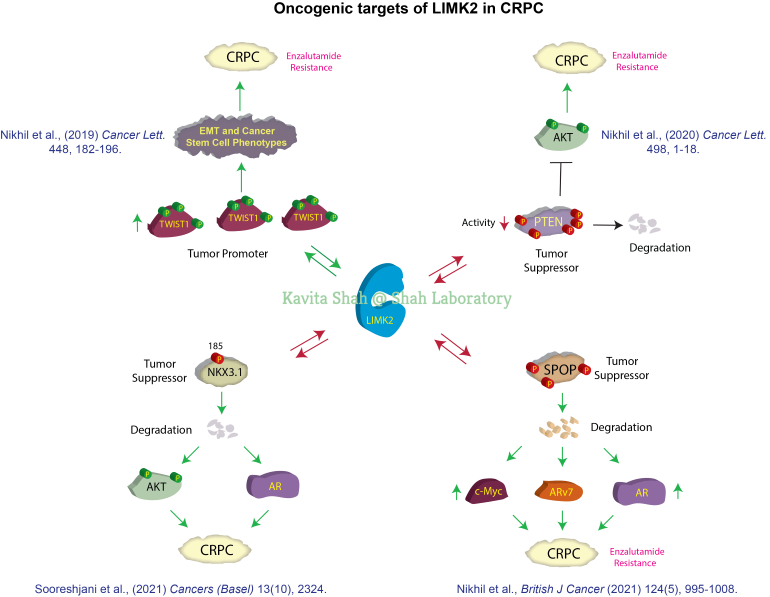
LIMK2 is predominantly a Ser/Thr kinase, although it can phosphorylate tyrosine as well. Under physiological conditions, it regulates actin dynamics by phosphorylating cofilin. LIMK2 is overexpressed in several human cancers and is strong associated with enhanced angiogenesis, tumorigenesis and metastasis. We previously discovered LIMK2 as a direct target of Aurora A kinase (AURKA). AURKA directly phosphorylates LIMK2 at three sites, which both stabilize and augment its kinase activity. In turn, LIMK2 directly binds AURKA and stabilize its levels. Thus, LIMK2 and AURKA feedback loop promotes highly aggressive oncogenic phenotypes. As AURKA is overexpressed in many human cancers, we postulated that LIMK2 may also be similarly overexpressed in these cancers. This lead to the identification of LIMK2 as a highly effective target for castration-resistant prostate cancer (CRPC). We show that LIMK2 depletion fully reverses tumorigenesis in mouse models. Despite this potential, the molecular mechanisms by which LIMK2 induces cancer is largely unknown as only a few LIMK2 substrates are known to date. We have recently discovered three downstream targets of LIMK2- PTEN, SPOP, NKX3.1 and TWIST1. While PTEN, NKX3.1 and SPOP are tumor-suppressors in prostate cancer, TWIST1 strongly promotes epithelial to mesenchymal transition and cancer stem cell phenotypes in CRPC. Identification of these substrates have unraveled several molecular mechanisms by which LIMK2 promotes CRPC pathogenesis.