Welcome to the Shah Laboratory
Drug Discovery in Cancer and Alzheimer's Disease Using Chemical Biology
Development of Novel Chemical Tools for Identification and Validation of G-Protein Targets in Cancer
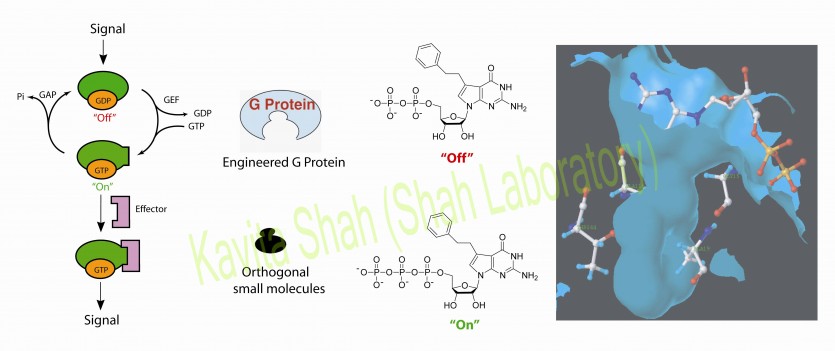
G proteins are a large family of proteins comprising approximately 0.5% of mammalian genomes. Binding of GTP or GDP locks G proteins into dissimilar ‘‘on’’ and ‘‘off’’ conformations, respectively, and thereby regulates their affinities to other proteins (effectors) and initiates signaling cascades. A wide variety of diseases are caused by deregulated G protein activities. Despite their great potential as drug targets, no active-site inhibitors are known to date. The high affinity of G proteins for their substrates (Kd 10 pM) and the high intracellular concentration of GTP (1 mM) have been blamed for this failure. The lack of such tools to allow for specific and temporal control over the activities of G proteins has prevented the contribution of small molecules to the elucidation of their pathways.
We have developed a chemical genetic approach for specific activation or inhibition of G Proteins. Small molecules that allow for the highly specific inhibition or activation of the engineered G protein were developed using H-Ras as a prototypical G Protein. The rational design preserved binding of the natural substrates to the G protein, and the mutations were functionally innocuous in a cellular context.
Furthermore, to ensure the transferability of this approach to other G proteins of interest, we demonstrated a straightforward transfer to Rap1B. As understanding the precise functions of closely related family members is a current frontier in Ras research, this specific control over the activity of a given member is of particular interest. More importantly, successful sensitization of a different G protein to the compounds controlling the activity of the previously engineered H-Ras demonstrates the potential breadth of application of this approach.