Design and Synthesis of Tunable Catalytic and Optoelectronic Nanomaterials
Overview. In the Li group, we are broadly interested in developing synthetic methods to precisely control the ensemble geometry and electronic properties of surface active sites in order access new modes of catalytic reactivity and selectivity over heterogeneous catalysts. In doing so, we have elucidated structure-activity and structure-selectivity relationships in electrochemical, thermal, and organic catalysis that will enable the rational design and precise synthesis of more efficient and selective materials catalysts.
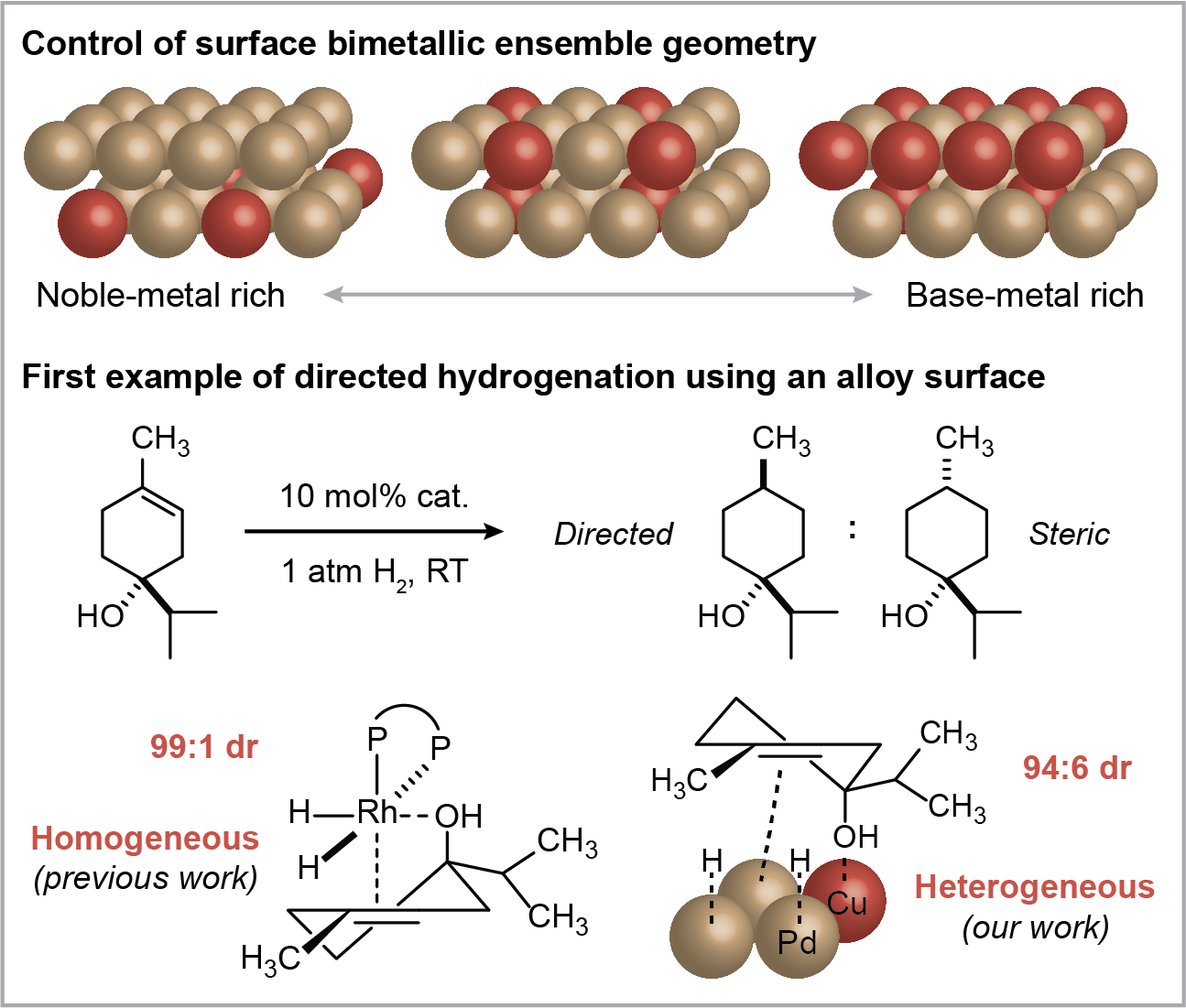
Heterogeneous Directed Hydrogenation
Our group recently developed a heterogeneous system to achieve stereoselectivity in organic hydrogenations using bifunctional binding to bimetallic alloy surfaces. We were inspired by the rich literature in molecular Rh and Ir complexes for substrate-directed hydrogenation, in which a heteroatom functional group on the substrate binds to the metal center and ‘directs’ hydrogen addition to the olefin from the same face. To apply this concept to a heterogeneous catalyst, we utilized bimetallic alloy surfaces containing both a noble metal and a base metal, where the base metal atom binds the directing group and adjacent noble metal atoms activate H2 and bind the olefin. Through careful control over thermal annealing conditions, we can access alloy nanoparticles with varying surface ensemble geometries, which has a dramatic effect on directed hydrogenation selectivity. We have now demonstrated several bimetallic catalysts (Pd-Cu, Pt-Ni) that are capable of hydrogenating tri- and tetrasubstituted olefins with high OH-directed diastereoselectivity (ACS Catal. 2021, 11, 6128-6134, Angew. Chem. Int. Ed. 2024, e202317710). While directed mechanisms are commonly observed in molecular catalysts, our work is the first example of hydroxyl-directed hydrogenation using a bimetallic heterogeneous catalyst. We believe that this brings us one step closer to achieving materials catalysts with orientational and electronic control comparable to molecular complexes (Perspective: ACS Catal. 2022, 12 , 7643–7654).
Nanoparticle Ligand Chemistry and Control of Surface Ensemble Geometry
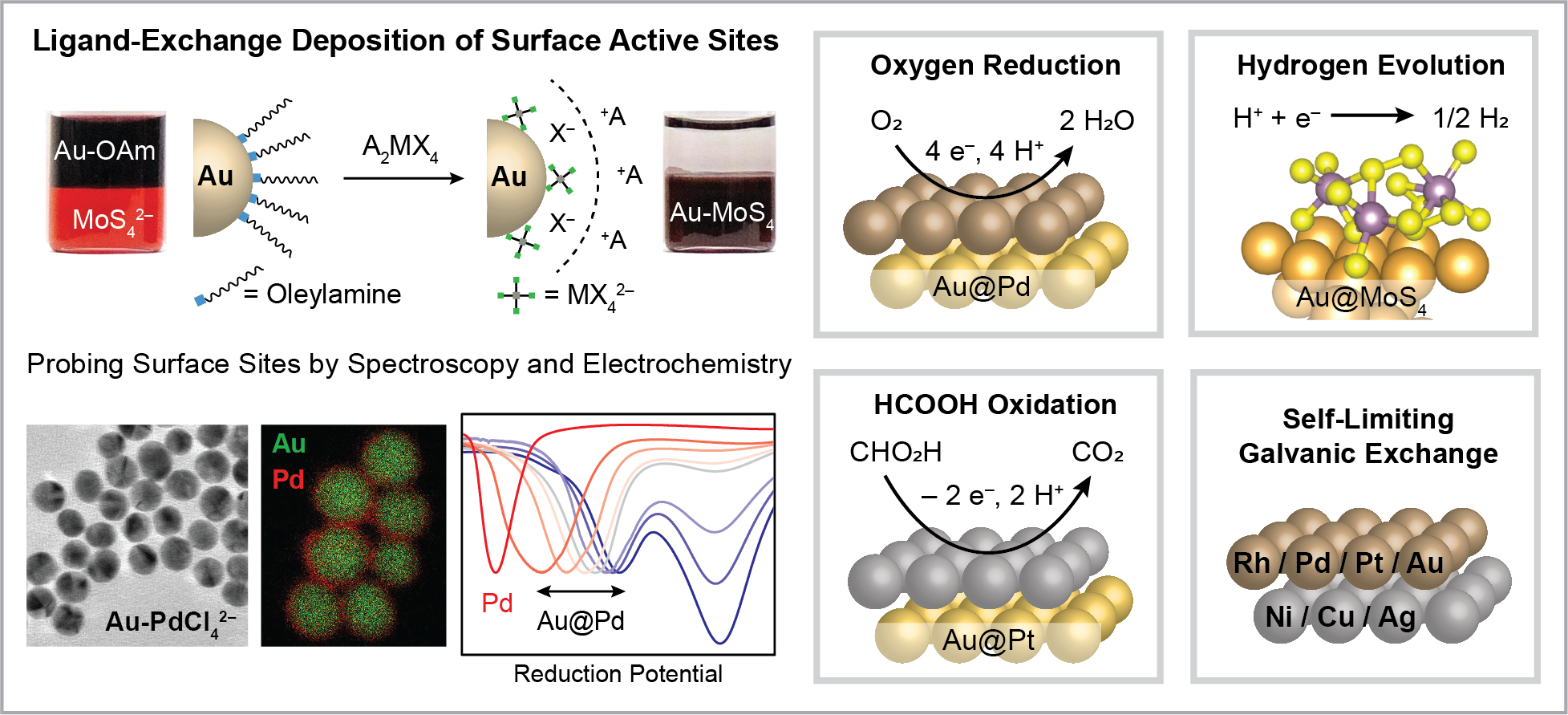
We are interested in inorganic nanoparticle ligands, specifically metal halides, metal oxides, and metal chalcogenides that can be utilized for catalytic applications. These ligands are capable of supporting colloidally stable particles in high dielectric solvents, and we have developed several colloidal ligand-exchange methods to generate core-shell structures with tunable electronic and geometric properties based on the interaction between the nanoparticle core and surface ligand.
We have utilized metal halide ligands to generate Au@Pd and Au@Pt nanoparticles for electrocatalytic oxygen reduction and formic acid oxidation (J. Am. Chem. Soc. 2018, 140, 28, 8918-8923, ACS Appl. Mater. Interfaces 2019, 11, 30977-30986) as well as bifunctional Pt nanoparticles coated with a thin layer of metal oxides for CO oxidation (Polyhedron 2019, 170, 239-244). We are also using anionic metal chalcogenides complexes as ligands, which serve as precursors to oligomeric structures that resemble the active sites in amorphous MoS2 on a molecular level. This project has been carried out in collaboration with Prof. Jeff Greeley’s group in chemical engineering, who has worked with us to computationally model how MoS4 interacts with our nanoparticle surfaces (ACS Catalysis 2020, 10, 13305-13313). To broaden the available compositions for the core nanoparticle, we are also developing self-limiting galvanic exchange reactions using organic ligands to tune the thermodynamic driving force (Chem. Mater. 2022, 34, 1987-1904). While a variety of ligands and cores are utilized in the above projects, we ask the same key question in every case: how can we use the interaction between the nanoparticle core and the surface ligand to enhance the catalytic properties of the material?
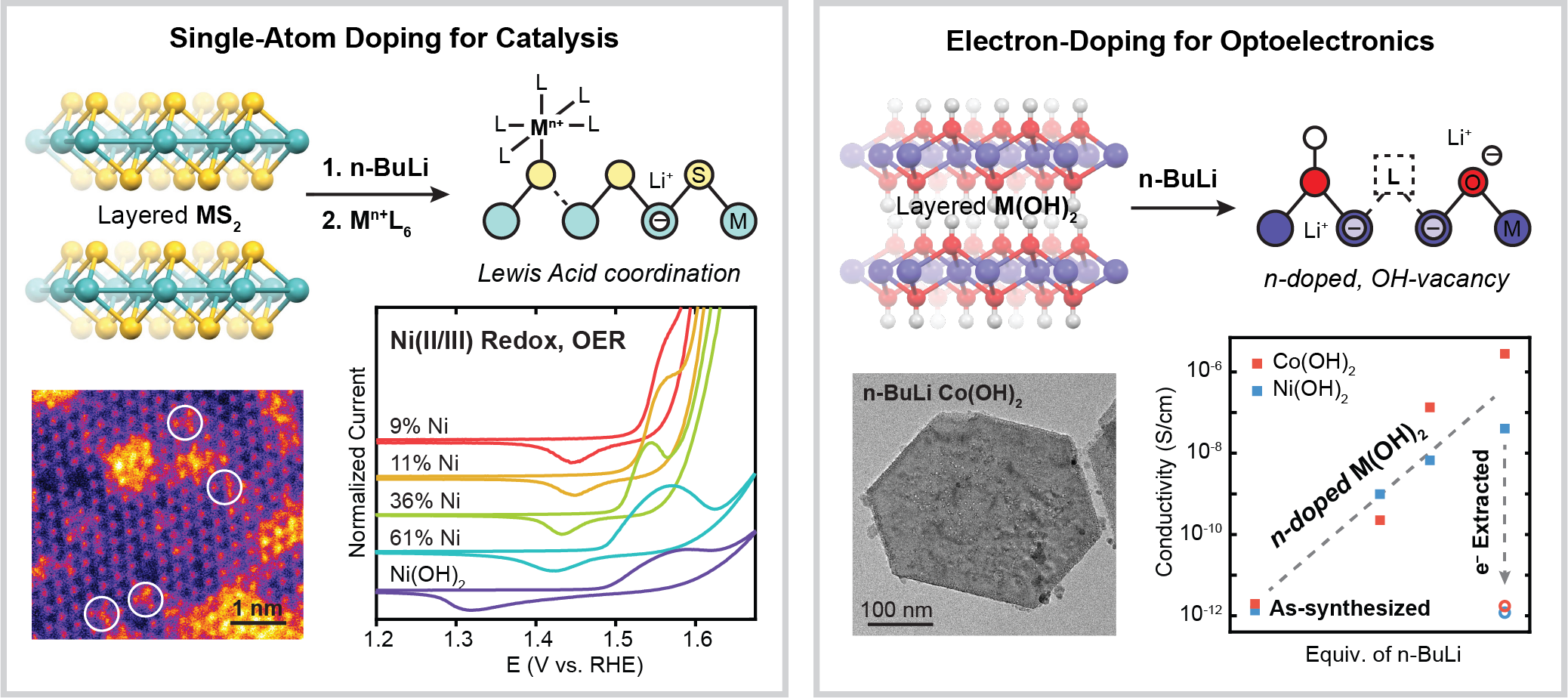
Doping of Colloidal 2D Materials
Another major effort in our group focuses on solution-phase doping of two-dimensional metal hydroxide and metal chalcogenide materials. We developed a chemical method to activate sulfide binding sites on the basal plane of colloidal WS2 nanosheets in order to functionalize the surface with Ni ions for oxygen evolution electrocatalysis (ACS Nano 2020, 14, 2238-2247). We are now expanding this methodology to other transition metal and post-transition metal dopants and additional electrocatalytic reactions (J. Mater. Chem. A 2021, 9, 19865-19873). Similar strategies can be employed on bulk WS2 microparticles for transition metal intercalation to enhance conductivity and carrier transport properties (Nano Lett. 2023, 23, 4471-4478, ACS Appl. Nano Mater. 2023, 6, 16846-16855). In a similar vein, we have utilized strong chemical reductants to electron-dope metal hydroxide nanosheets in order to tune their electronic and conductivity properties. We have focused on understanding how the layered structure of the metal hydroxide material enables stable electron storage (Nano Lett. 2020, 20, 7580-7587, Inorganic Chemistry 2021, 60, 6950-6956) and plan to expand these methods towards transparent conducting oxides and electrochromic materials.