Philip S. Low
Next Generation Targeted Therapeutics
Development of a novel CAR T cell immunotherapy for cancer
Patient-derived T cells are now being genetically engineered to attack cancer cells more aggressively and specifically. While remarkable improvements have been achieved in treating hematologic cancers, chimeric antigen receptor (CAR) T cell therapies of solid tumors remain encumbered by several limitations, including:
- Tumor heterogeneity leading to selection for antigen-deficient cancer cells
- Induction of cytokine storms with potentially lethal consequences,
- CAR T cell exhaustion deriving from chronic exposure to tumor antigens,
- An inability to manipulate CAR T cell activity (e.g. terminate cell killing) following infusion into the cancer patient
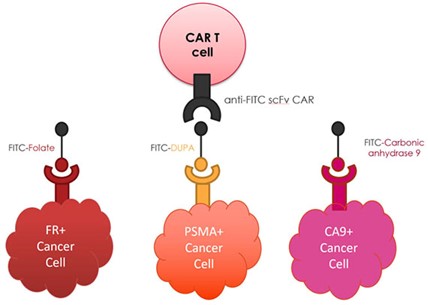
We have developed a universal CAR T cell therapy that solves each of these problems by employing a low molecular weight bispecific adapter to bridge between the CAR T cell and cancer. In this strategy, the CAR T cell is engineered to recognize the yellow dye fluorescein and the bispecific adapter is constructed with fluorescein linked via a spacer to a small molecule cancer cell-specific binding ligand. Cancer cell killing is then initiated by administration of the bispecific adapter (see above diagram) that forms a bridge between the anti-fluorescein CAR T cell and cancer cell, thereby inducing CAR T cell killing of the cancer cell. We have shown that the rate and specificity of this cancer cell killing can be sensitively controlled by adjusting the dosing frequency, dosing concentration and tumor specificity of the bispecific adapter. Furthermore, we have demonstrated that a cocktail of bispecific adapters can enable our universal anti-fluorescein CAR T cell to engage multiple antigenically distinct cancer and stromal cells simultaneously, leading to their concurrent destruction. Most importantly, those CAR T cells that become exhausted due to chronic antigen exposure can be rapidly rejuvenated by interrupting bispecific adapter administration for a short period (see representative publications).
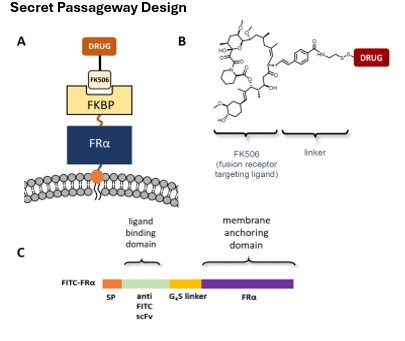
Sensitive control of cell-based therapies
Cell-based therapies are being explored for treatment of a variety of diseases, with applications expected to rise as methods for engineering, editing, and proliferating human stem, progenitor and more differentiated cells are refined. While some obstacles to cell-based therapies have been solved, concerns remain regarding our inability to terminate, inhibit, proliferate, activate or differentiate these cells after their infusion into a patient. Because engineered cells can behave in unwanted ways, methods to rapidly and sensitively control their functions following transplantation may eventually be required to avoid black-box labels.
Our lab has developed a sensitive method to control the properties of any cell-based therapy following its infusion into a patient. In this method, a “secret passageway” protein is inserted into the therapeutic cell before its administration to the patient (see cartoon below). Because this totally human FKBP-linked fusion protein is designed to continually internalize, recycle through endosomes and return to the cell surface, it repetitively facilitates the entry of FK506-linked drugs (e.g. drugs, genes, proteins, RNAs, antibodies, etc.; see diagram in panel B below) into any cell that has been transduced with the secret passageway. Because the FK506-linked drug is designed to avoid entry into any cell that lacks the secret passageway and release an unmodified drug following internalization by the engineered cell, the technology can be used to modulate any cell-based therapy without perturbing the behavior of any other cell in the body. Applications of this secret passageway have included suppression of a cytokine storm, reactivation of exhausted CAR T cells, elimination of CAR T cells when their target cells have been eradicated, and stimulation CAR T cell proliferation in vivo. Anticipated applications further include controlled differentiation of stem cells following their transplantation into a host and targeting of stem/progenitor cells to a specific tissues/organs. In brief, the “secret passageway” enables sensitive control of any cell-based therapy after its infusion into the patient.