Philip S. Low
Next Generation Targeted Therapeutics
Structural characterization of the human red blood cell membrane
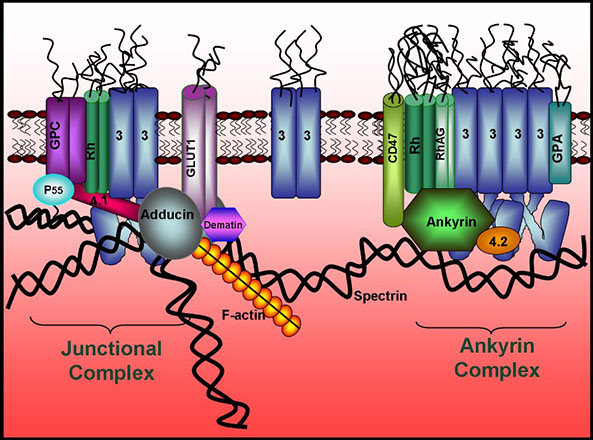
We are studying the structure and function of the human erythrocyte membrane and its role in human health and disease. Because the membrane-spanning protein, band 3, catalyzes anion transport, links the cortical cytoskeleton to the membrane, organizes a glycolytic enzyme complex on the membrane, binds and regulates at least 8 other proteins, and serves as the senescent cell antigen, we are characterizing its structure and function at the molecular level. We are also investigating the roles of other membrane proteins in controlling cell shape, regulating erythrocyte metabolism, modulating cell flexibility and stability, and mediating oxygen delivery. In the course of these studies, we have demonstrated that tyrosine phosphorylation of the cytoplasmic domain of band 3 (cdb3) regulates many RBC properties, including glucose metabolism, anion transport, and membrane stability. We have further shown that the mechanism by which this phosphorylation regulates RBC properties involves docking of the tyrosine phosphorylated cytoplasmic domain of band 3 with a membrane exposed SH2-like (MESH) domain within the membrane-spanning domain of band 3. We are now examining the role of these MESH domain interactions in the pathologies of malaria and sickle cell disease.