The Ghosh Laboratories:
The Home of the Backbone Binding, Bioactive Natural Product Synthesis, and Nature-Inspired Molecular Design for Today's Medicine
Ghosh Group »
Research »
Total Synthesis of Bioactive Natural Products
Total Synthesis of Bioactive Natural Products
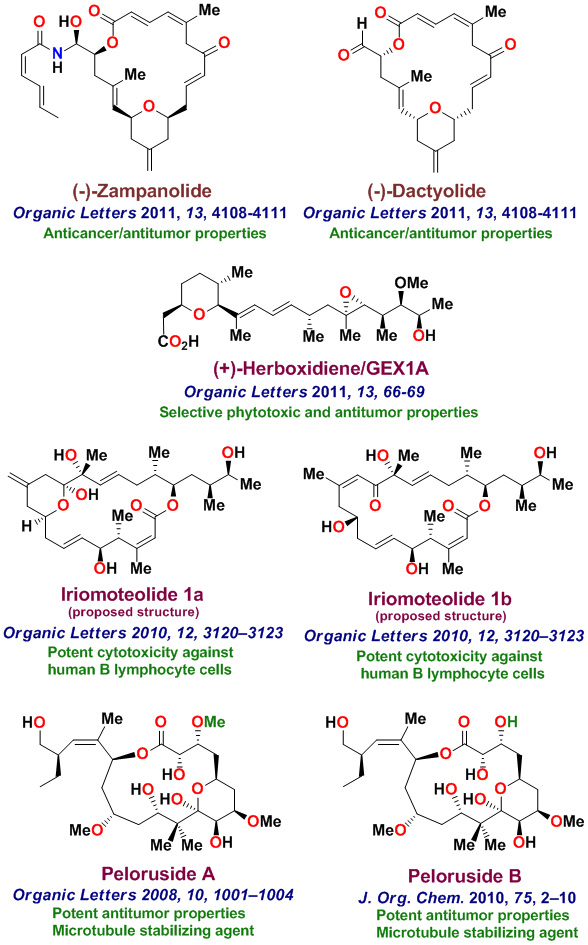
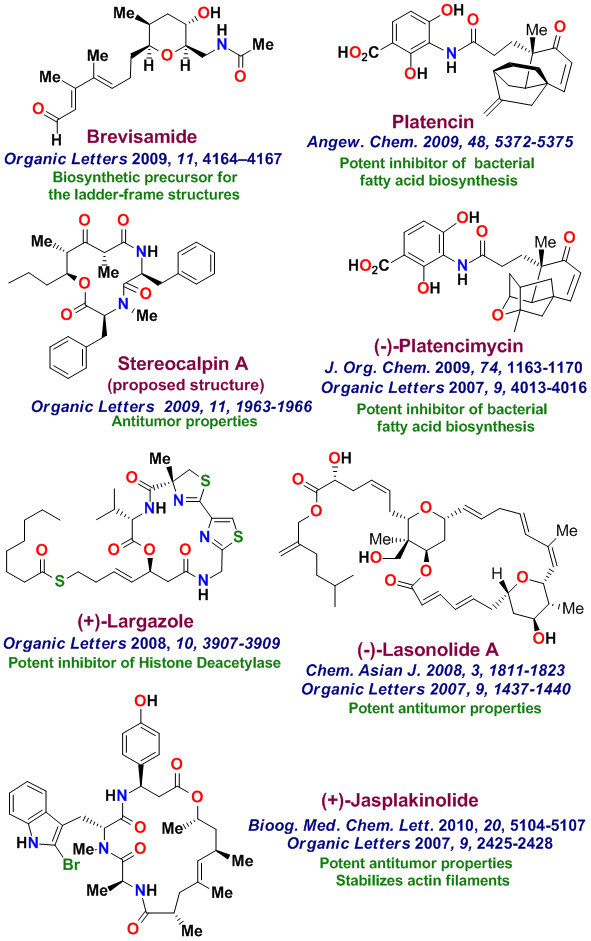
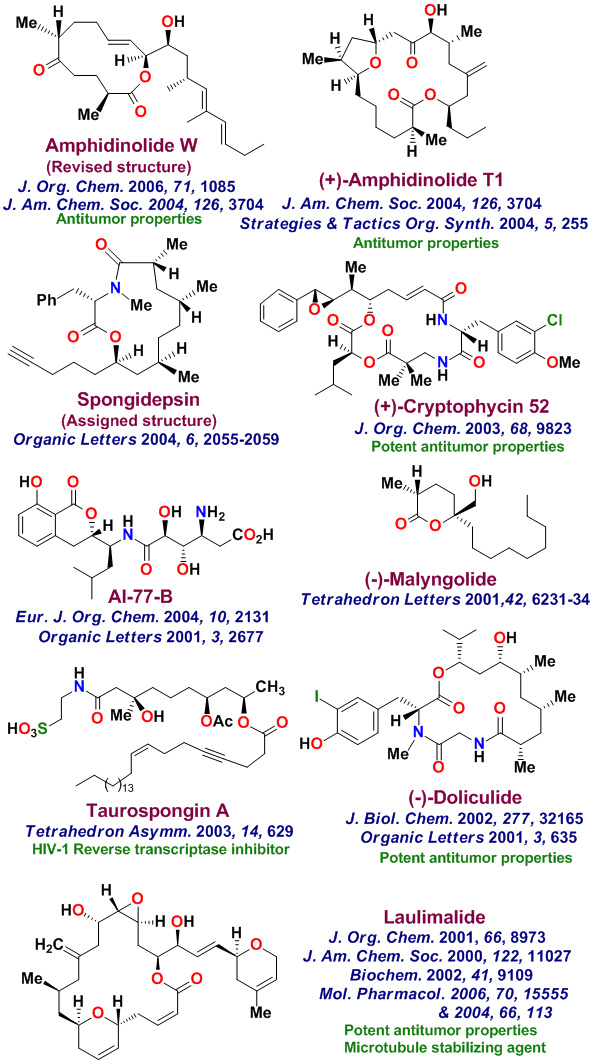
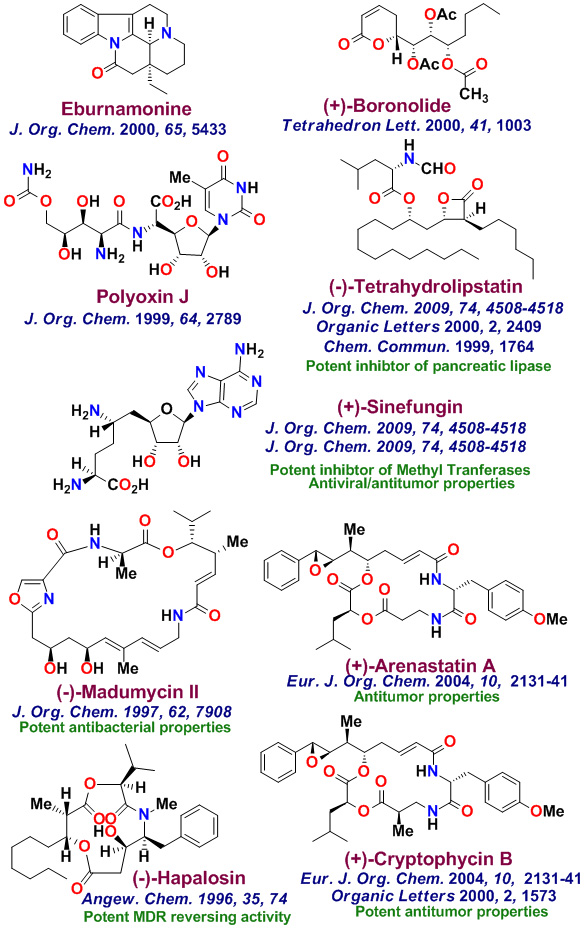
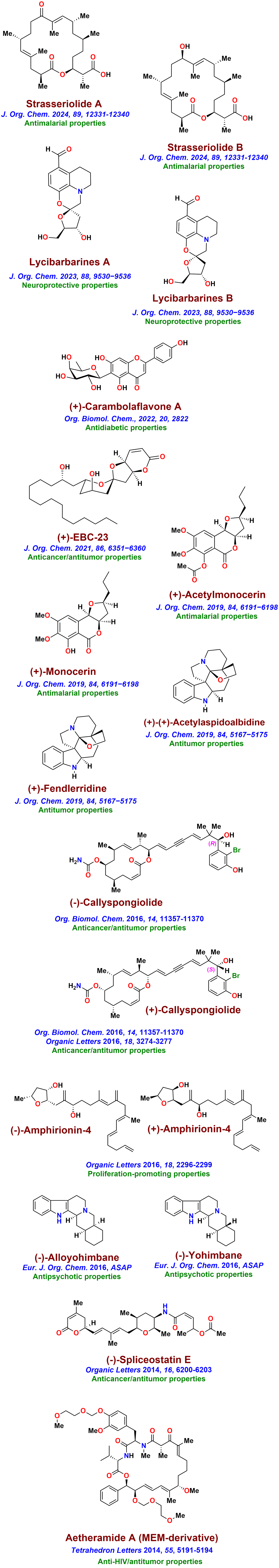
The total synthesis of various biologically important natural products is an important part of my group's research. Our underlying interests include the development of new synthetic methodologies as well as the opportunity to establish important structure-function relationships for these rare natural products. with medicinal significance. We have synthesized a variety of complex natural products with significant medicinal potential. To date, our numerous bioactive natural products with distinct structural classes, were synthesized in our laboratories. These Bioactive molecules are shown below. Laulimalide, a sponge-derived macrolide exhibited very potent anticancer properties, however it was isolated in only miniscule quantities. Our chemical synthesis of Laulimalide enabled us to carry out further biological studies in collaboration with Dr. Ernie Hamel at the National Cancer Institute. While laulimalide was thought to resemble paclitaxel in its effects on cellular microtubules, our studies established that laulimalide stabilizes microtubules by binding at a novel site on the tubulin dimer. Laulimalide appears to be the first example that binds to different drug-binding site on tubulin. Our work established that laulimalide enhance tubulin assembly synergistically with paclitaxel. Furthermore, we showed that the epoxide functionality is not essential for activity. Our synthetic derivative Desoxylaulimalide displated similar potency as laulimalide. We have also demonstrated that Peloruside A, Peloruside B, and Zampanolide are potent anticancer agents and they exhibit microtubule stabilizing properties. We have carried out efficient syntheses of several other important anticancer natural products, investigated in-depth biology, and synthsized various structural variants for SAR studies. Our enantioselective synthesis of Doliculide enabled us to establish its biological mechanism of action.
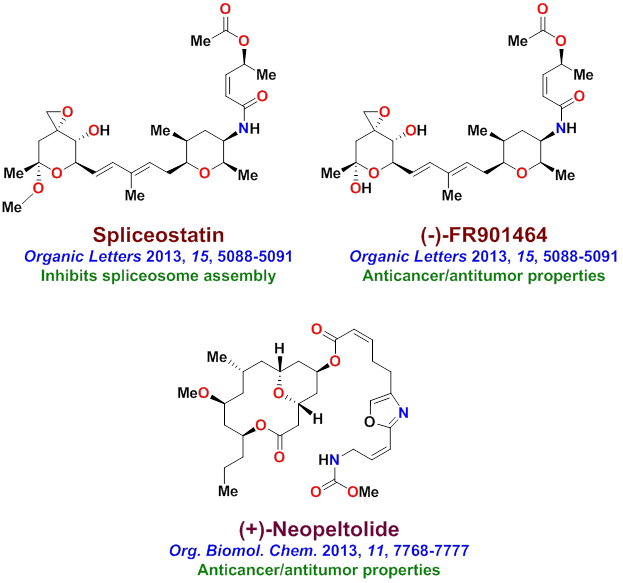
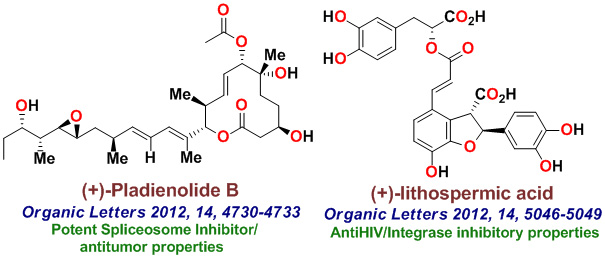
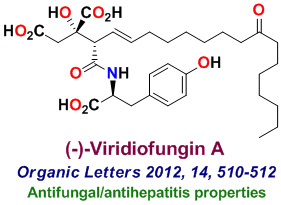
Our research group completed the total synthesis of many other biologically important natural products including, anticancer agents such as Amphidinolide T, Amphidinolide W, Spongidepsin, Callyspongiolide, Cryptophysin B, Cryptophysin 52, Dactylolide, Doliculide, EBC-23, and Neopeltolide, MDR- inhibitor Hapalosin, streptogramin antibiotic Madumycin, pancreatic lipase inhibitor, tetrahydrolipstatin, antimalarial agents Boronolide, Monocerin, Acetylmonocerin, Strasseriolides A and B and gastroprotective agent, AI-77-B, reverse transcriptase inhibitor, taurospongin, nucloside antibiotic sinefungin, polyoxin-J; HDAC inhibitor, largazole, anti bacterial agents, platencin, platensimycins, and herboxadine. Following the synthesis of (-)-lasonolide A, in collaboration with Dr. Yves Pommier at the NCI, we showed that lasonolide possesses a novel chromosome condensing ability. Subsequently, using synthetic lasonolide and its structural variants, we investigated their biological mechanism of action. Furthermore, we carried out enantioselective synthesis of antifungal natural products, viridofungin, and antitumor natural products, herboxidiene, pladienolide, spliceostatins, and FP901464. These are very potent inhibitors of spliceosome. Our work on pladienolide, herboxidiene, and spliceostatins allowed the establishment of structure-activity studies, X-ray structural studies with spliceosome, and design of less complex inhibitors for cancer chemotherapy.