Research
Metalloproteins are important in biology because they catalyze essential reactions in life like cellular respiration, photosynthesis, and nitrogen fixation under mild conditions and with high efficiency and selectivity. Research in the Tian lab lies at the interface of physical, inorganic, and biological chemistries and utilizes a wide range of spectroscopic and computational techniques to elucidate the mechanisms of metalloproteins. The information gained through this type of work will provide insight into the electronic and geometric structures of metalloproteins and their associated mechanistic roles in pathogenesis, which can be beneficial in the development of effective therapeutic treatments.
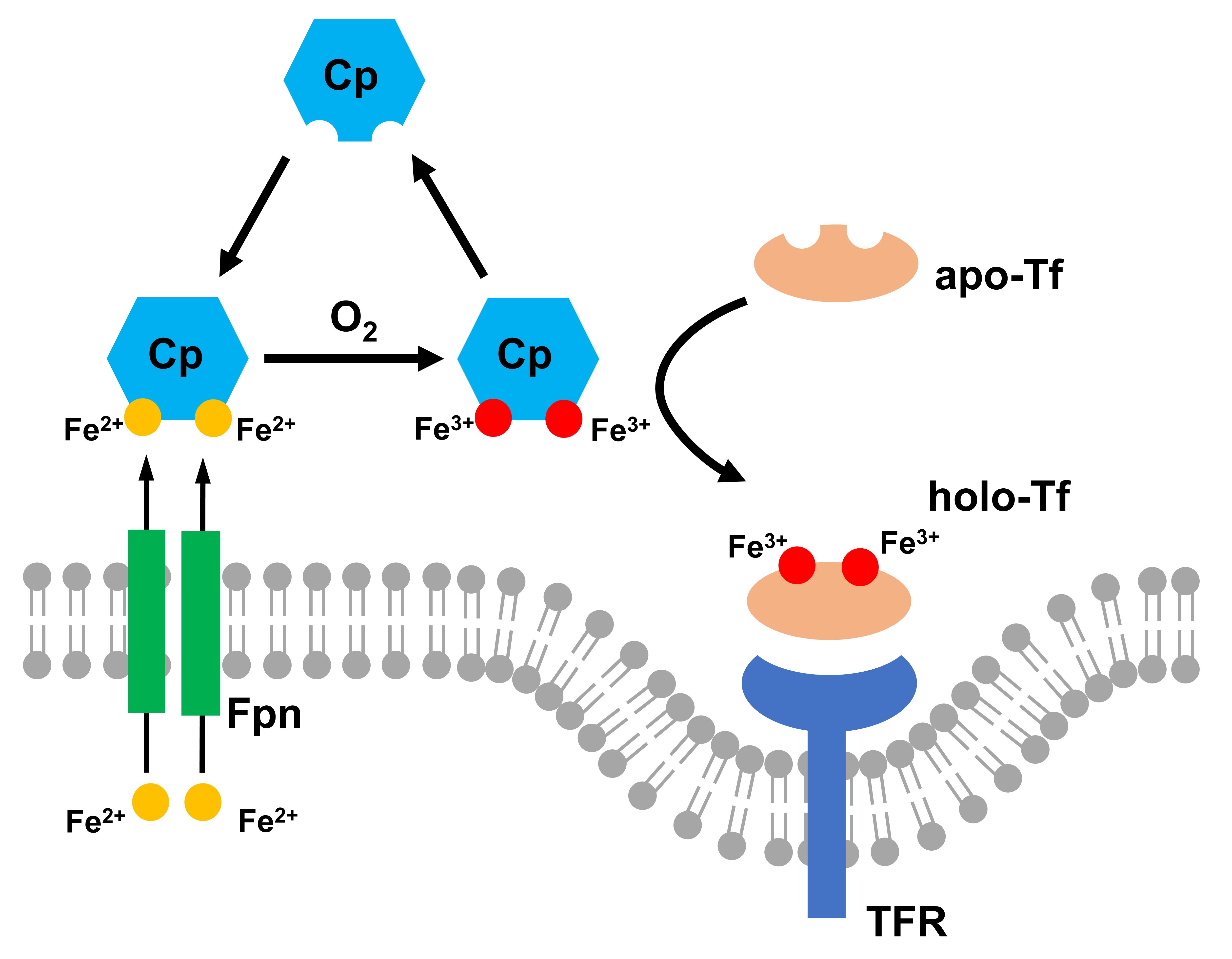
1. Iron Homeostasis in Neurodegenerative Diseases
Fe is a redox-active metal essential for the regulation of cellular pathways that are fundamental for brain function, including oxygen transportation, DNA synthesis, mitochondrial respiration, myelin synthesis, and neurotransmitter synthesis and metabolism. Fe is tightly regulated by sophisticated homeostatic systems that tune its level and localization. Perturbation of this regulation is evident in the brain affected by neurodegeneration. The accumulation of Fe is consistently observed in the parietal cortex, motor cortex, and hippocampus of Alzheimer’s patient brains. Thus, Fe plays a critical factor in the development of neurodegenerative diseases. While most of the current research focuses on Fe accumulation induced increase of oxidation-stress and neurotoxicity through ROS production, our lab aims to understand the correlation of pathological causes of neurodegeneration to the dysfunction of metalloproteins and dysregulation of Fe homeostasis.
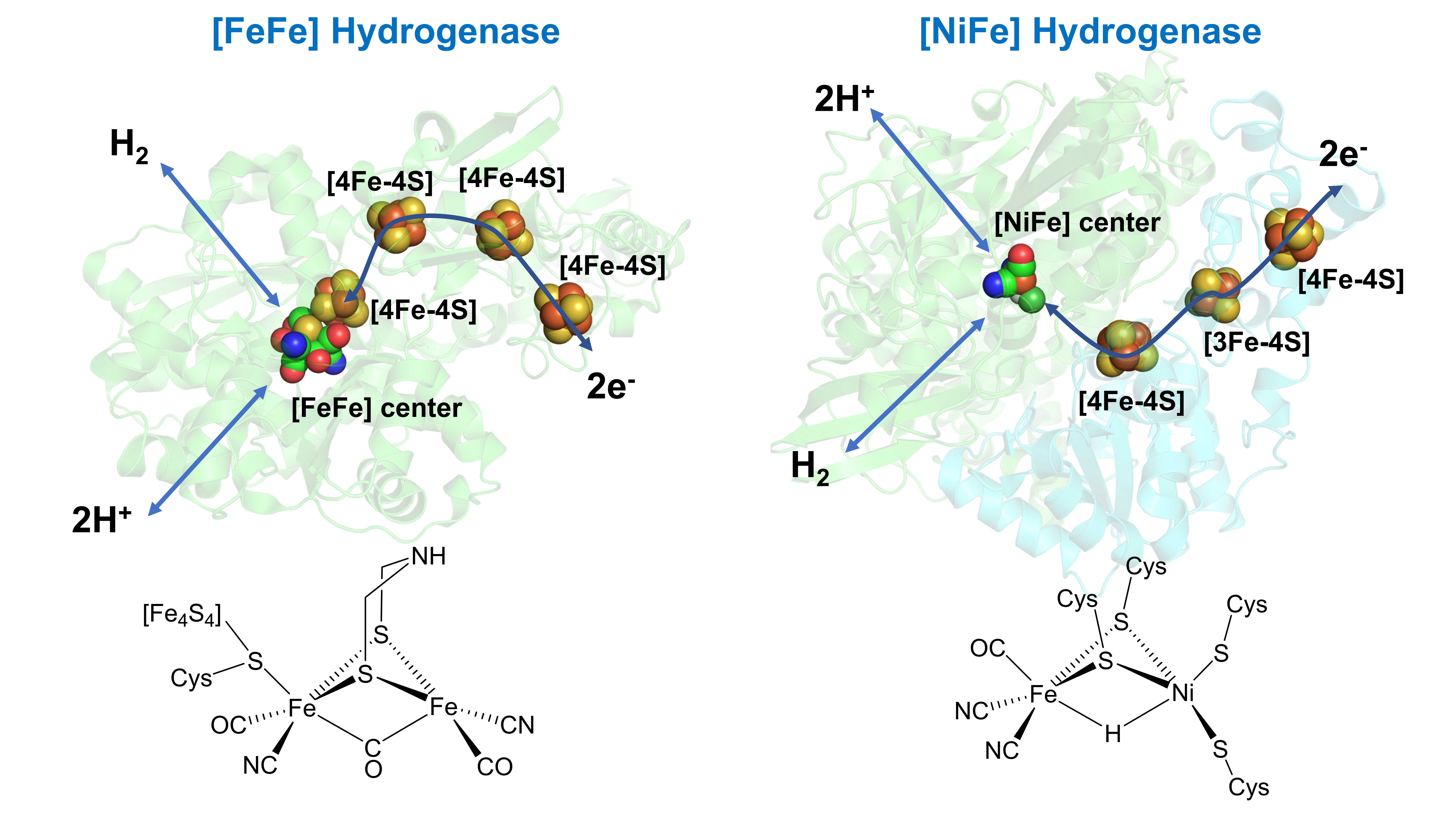
2. Metalloprotein Design
The observation that nature achieves diverse functions using a limited number of scaffolds suggests that instead of designing a new scaffold for every new function, it is possible to use naturally evolved proteins as scaffolds to design and engineer various new structures and functions. Such a protein redesign strategy can bypass the challenge of developing a stable protein fold because many native proteins possess robust tolerance towards multiple mutations. Redesign of small, stable, easy-to-make, and well-characterized proteins as scaffolds to mimic the functions of large, multi-domain, membrane proteins has been proven to be an effective way of metalloprotein engineering. Our lab seeks to reconstruct the active sites of [FeFe] and O2-tolerant [NiFe] hydrogenases in attainable protein scaffolds. A robust, environmentally benign, and efficient artificial hydrogenase provides new opportunities to sustainably produce H2 and replace fossil fuels in the future to tackle the climate problem by decreasing the emission of CO2.
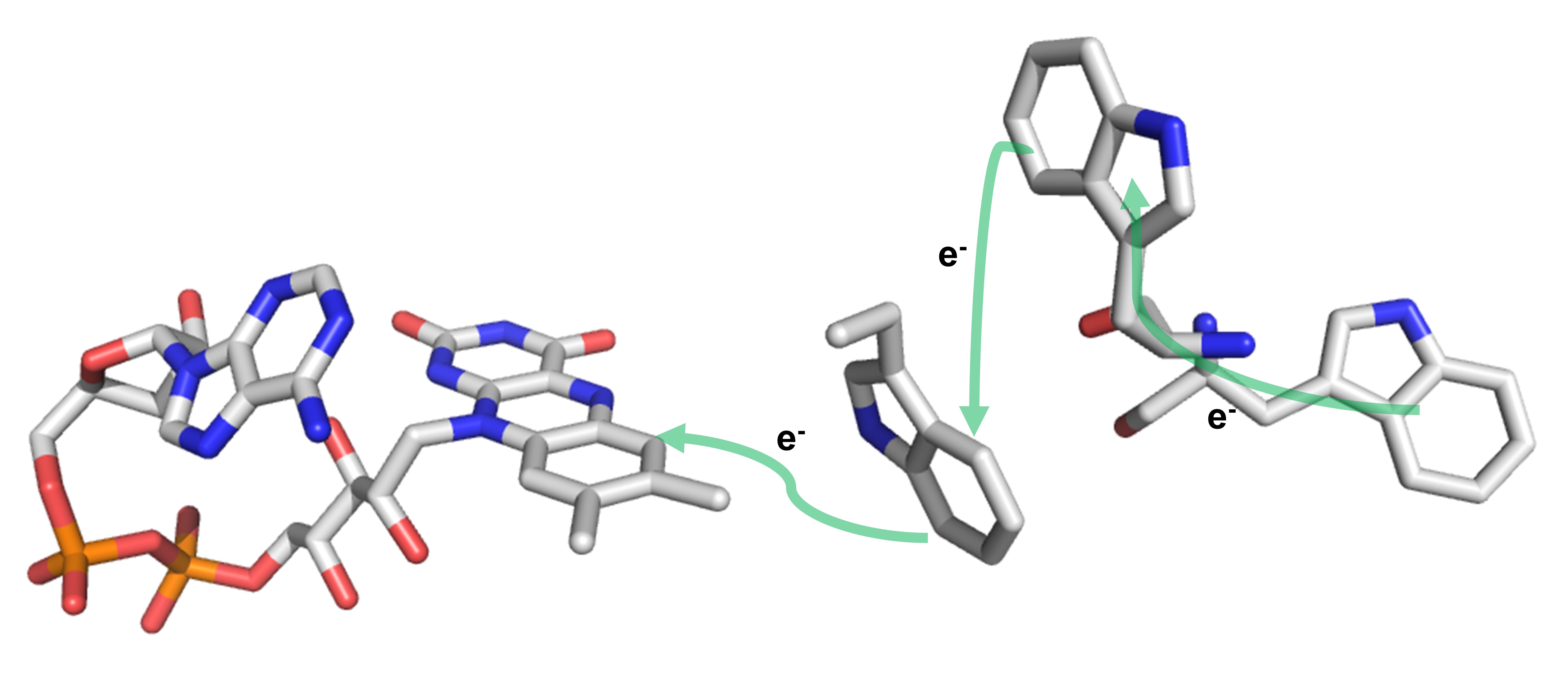
3. Magnetoreception
Although it has been well known that migratory birds can sense the earth’s magnetic field to navigate their migrations, the biological nature of such a magnetic sensing mechanism remains unclear. The most accepted hypothesis is that a pair of flavosemiquinone and tryptophan radicals are generated by photoexcitation of cryptochrome proteins, so-called the radical-pair mechanism. But such a hypothesis does not answer the question of night-time navigation. Also, the cryptochrome itself cannot form a biocompass. Thus, the link between a radical-pair mechanism and migratory birds sensing the direction of the earth’s magnetic field is still missing. We will spectroscopically characterize the metalloproteins identified by bioinformatic studies that potentially interact with cryptochromes to elucidate the molecular basis of the mechanism of magnetoreception.