Gas-Phase Pyrolysis by Charge-Remote Fragmentation
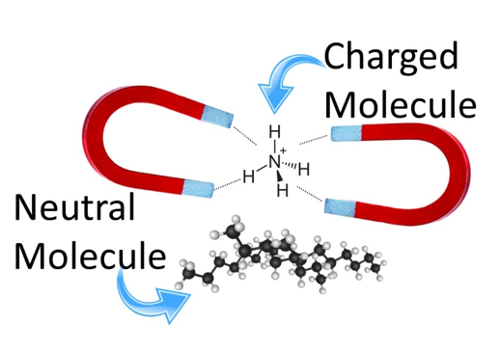
Charge-remote fragmentation is a technique in mass spectrometry used for studying the thermolysis of neutral molecules in the gas phase1. Normally, neutral molecules go undetected in mass spectrometry because it relies on ions holding a charge so that they can be manipulated through the instrument using electric and magnetic fields (Figure 1). Because they are not charged, neutral molecules do not reach the detector and simply fall out of the instrument. Charge-remote fragmentation overcomes the inability of mass spectrometers to detect neutral molecules by attaching a charged molecular tag to them.
Figure 1. A charged molecule (NH4+) being suspended by magnets. Conversely, neutral molecules cannot be manipulated by magnetic fields because they are not charged
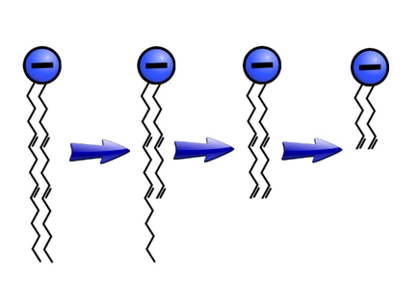
This technique is useful for studying the structures of biological molecules, such as lipids. Lipids have long, non-polar tails with a charged polar head. Determining the connectivity of the bonds in the tail, which can be arranged in many ways, is a challenge. Using charge-remote fragmentation, lipids break apart into smaller and smaller pieces, which are then detected by the mass spectrometer. Reverse engineering the fragments back into the original molecule reveals how the bonds in the original molecule were connected (Figure 2).
Figure 2. Structural analysis of a lipid using sequential charge-remote fragmentation. The length of the fragments that break off help reveal the locations of the double bonds.
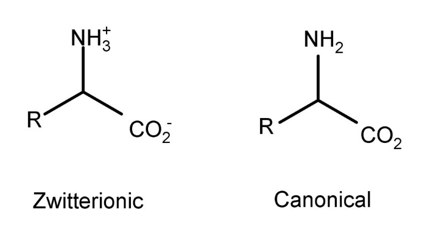
In our lab, we have applied this technique to study the thermolysis of the canonical or neutral structure of amino acids2. In a solvent, amino acids exist as zwitterions, but in the gas phase amino acids would be non-ionic (Figure 3). The canonical structure of an amino acid is difficult to study using mass spectrometry because traditional methods for ionizing the molecule would change it from its canonical form. By attaching a charged molecular tag to the R groups of amino acids, we can preserve the canonical nature of amino acids and see what happens to them as they undergo thermal degradation.
Figure 3. The ionic and canonical forms of amino acids.
Currently, we are applying charge-remote fragmentation to study the mechanisms involved in the pyrolysis of lignin, a biopolymer that grows in the cell walls of plants. Lignin is a renewable resource of aromatic compounds, yet it has always been a challenge to study because of its large size and lack of ionizable groups. High heat must be used to volatilize lignin to get it into the gas phase, so pyrolysis chambers or even lasers are often used. We hope to bypass lasers and pyrolysis chambers by attaching charged groups to lignin, which will help volatilize lignin and as well as allow for charge remote fragmentation. By studying how lignin breaks down in the gas phase, we hope to uncover mechanisms of its conversion into more valuable chemicals.
References
-
J. Adams, M. L. Gross, J. Am. Chem. Soc. 1989, 111, 435.
-
D. Koirala, S. R. Kodithuwakkuge, P. G. Wenthold, J. Phys. Org. Chem. 2015, 10, 635-644