Toward an Open-Shell Singlet Nitrene
Nitrenes are one of the most widely studied reaction intermediates with unfilled electronic valences that lead to their high reactivity. Although, both the phenyl carbenes and phenyl nitrenes are isoelectronic with ground-state triplets, phenyl carbene has a closed shell lowest energy singlet state σ2 whereas, phenyl nitrene has an open shell lowest energy singlet state, σπ. The singlet nitrene, if not trapped, eventually undergoes inter-system crossing (ISC) to the triplet state. For phenyl nitrene, ISC from σπ singlet state to the triplet is slow. As a consequence, various other processes competitive to ISC can emerge. This has proven to be a huge challenge in harnessing the reactivity of the nitrenes.
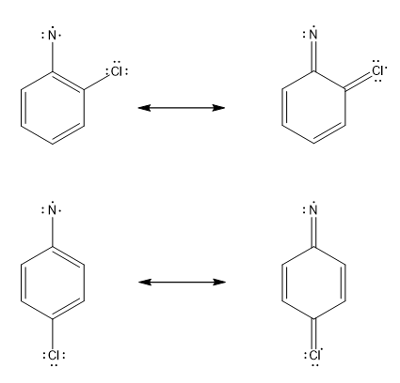
The open shell singlet state of these phenyl nitrenes can only be influenced by the radical stabilizing properties of the substituents. However, not many substituents are capable of significantly affecting the radical stability. Studies have shown that appropriate substitution at ortho or para positions cause separation of the electrons in the open shell singlet of phenyl nitrenes by forming quinoidal resonance structures, thereby stabilizing the open shell singlet nitrene.
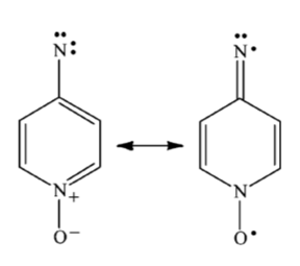
It has also been demonstrated from the study of pyridinyl N-oxide nitrenes that, the phenyl nitrene radical can be stabilized by delocalization onto the oxygen, essentially creating a nitroxyl radical, which is generally considered a highly stable free radical.
The formation of these stable radicals although, comes at the cost of the aromatic stabilization energies of the moieties which need to be compensated for.
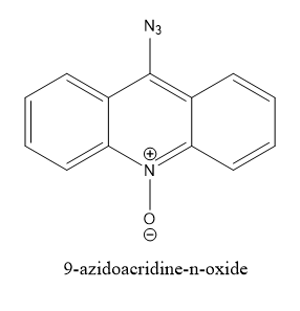
Considering all these factors and the challenges, we aim at building a suitable electronic environment that can stabilize the open shell singlet of the phenyl nitrene. Utilizing a highly aromatic backbone with appropriate substitution should substantially reduce the singlet-triplet splitting in a nitrene and promote ISC which would help in exploiting the reactivity of a nitrene. An example of a molecule that we are currently working on is the 9-azidoacridine-n-oxide.
This project involves synthesis of the nitrene moieties followed by laser flash photolysis studies of the same.
References
-
Gritsan, N. P.; Platz, M. S. AdV. Phys. Org. Chem. 2001, 36, 255– 304
-
Wijeratne, N. R.; Wenthold, P. G. J. Phys. Chem. A. 2009, 113, 9467–9473
-
Wenthold, P. G. J. Org. Chem. 2012, 77, 208−214