Mass Spectrometric Detection of Phenols using the Gibbs Reaction
Phenols are ubiquitous in our world. It is found all types of natural and non-natural system. For example, it occurs naturally such as tyrosine, capsaicin, morphine, tetrahydrocannabinol, red wine tannins, etc. Anthropogenic sources such as industrial waste have high phenol concentration. To quantify it, many chromatographic methods have been used since many years1. In our lab, we are making derivative of phenols, indophenols, before mass spectroscopic analysis (Eq. 1).
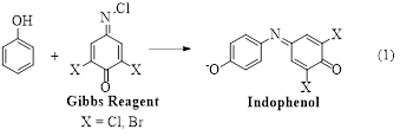
The key feature that makes indophenol a useful approach for the detection of phenols is its robust color which allows for easy spectrophotometric detection2. However, the spectrophotometric absorption peak of substitute phenols is nor very sensitive3, 4. Consequently, this technique is generally not capable of distinguishing between individual substituted phenols. Thus, a separation step is required for the detection of specific phenol derivatives5. On the other hand, indophenols can also be detected by electrochemistry and cyclic voltammetry. In our lab, we are using simple electrospray ionization mass spectrometry (ESI-MS) for the detection of indophenols. Since indophenols are naturally anionic, they are readily been detectable by ESI-MS6. The readily distinguishable nature of indophenols of different substituents of phenols makes the use of mass spectroscopy important. For example, by using our technique, it is easily distinguished and eventually quantify the different phenols in commercial liquid smokes (Fig. 1). In addition, the deconvolution method of chlorin or bromine isotopic peaks confirm the presence of indophenols. The following figure is the deconvoluted mass spectra of the Gibbs product of Natural Hickery and Naturul Mesquite flavors of Colgine brand liquid smoke. The presence of m/z 282, 296, and 326 are due to the presence of catechol, guaiacol, and syringol, respectively6.
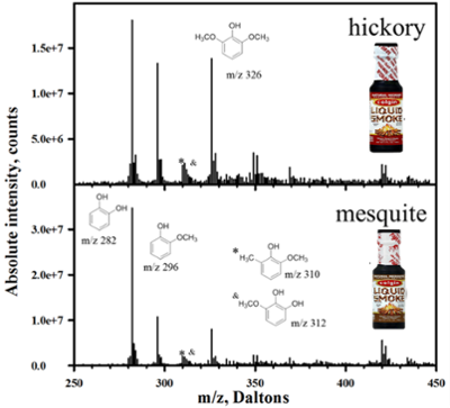
Figure 1. Deconvoluted mass spectrum of the Gibbs products obtained from analysis of Natural Hickory (top) and Natural Mesquite (bottom) flavors of Colgin brand liquid smoke6.
References
-
Arutchelvan, V.; Kanakasabai, V.; Nagarajan, S.; Muralikrishnan, V., Isolation and identification of novel high strength phenol degrading bacterial strains from phenol-formaldehyde resin manufacturing industrial wastewater. Journal of Hazardous Materials 2005, 127 (1-3), 238-243.
-
Brooker, L.; Sprague, R., Color and constitution. IV. 1 The Absorption of phenol blue. Journal of the American Chemical Society 1941, 63 (11), 3214-3215.
-
Ryu, W.-K.; Kim, H.-W.; Kim, G.-D.; Rhee, H.-I., Rapid determination of capsaicinoids by colorimetric method. journal of food and drug analysis 2017, 25 (4), 798-803.
-
Montazeri, N.; Oliveira, A. C.; Himelbloom, B. H.; Leigh, M. B.; Crapo, C. A., Chemical characterization of commercial liquid smoke products. Food science & nutrition 2013, 1 (1), 102-115.
-
Lowe, E. R.; Banks, C. E.; Compton, R. G., Indirect detection of substituted phenols and cannabis based on the electrochemical adaptation of the Gibbs reaction. Analytical and bioanalytical chemistry 2005, 383 (3), 523-531.
-
Mistry, S.; Wenthold, P. G., Mass spectrometric detection of the Gibbs reaction for phenol analysis. Journal of Mass Spectrometry 2018.
