Alzheimer's drug based on Purdue-designed inhibitor begins clinical trials
2007-06-25
A drug based on the design of a Purdue University researcher to treat Alzheimer's disease began the first phase of human clinical trials this week.
"Millions of people suffer from this devastating disease and treatment options are very limited," said Arun Ghosh, the Purdue professor who led the creation of the treatment molecule. "Current drugs manage the symptoms, but this could be the first disease-modifying therapy. It may be able to prevent and reverse the disease."
CoMentis Inc., a biopharmaceutical company based in San Francisco, is initiating the clinical trials of the experimental drug CTS-21166. Ghosh, who has dual appointments in the departments of chemistry and medicinal chemistry, is a scientific co-founder of the company with Jordan Tang, the J.G. Puterbaugh Chair in Medical Research at the Oklahoma Medical Research Foundation.
The collaborative work of Ghosh and Tang led to the development of a treatment that could intercept and disable the disease at an early stage.
In 2000, Tang identified beta-secretase, a key enzyme in the progression of Alzheimer's that triggers the formation of amyloid plaques in the brain. Various stages in plaque formation produce toxic proteins that harm the brain, causing damage that eventually leads to dementia.
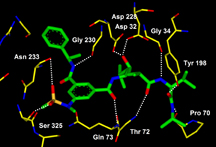
Later that year, Ghosh built a molecule that binds to this key enzyme and inhibits its activity, a beta-secretase inhibitor.
"These molecules fit together like puzzle pieces," Ghosh said. "We created a molecule that fits with a key piece of the Alzheimer's disease puzzle. When the treatment mole
Xiaoming Xu (l) and Arun Ghosh (r)