Lithium Reinvented: A simple conversion could spark an energy revolution
2025-08-14
Researchers at Purdue University use mass spectrometry to convert lithium salts to metal nanoparticles
The race is on to develop alternative methods for producing lithium metal, a critical material for today’s emerging battery technologies. Purdue researchers found a simple water-droplet-based method to turn common lithium salts into high-energy lithium metal using mass spectrometry.
The team, led by Graham Cooks, Henry Bohn Hass Distinguished Professor in the James Tarpo Jr. and Margaret Tarpo Department of Chemistry at Purdue University, recently published their patent-pending breakthrough in the Royal Society of Chemistry journal Chemical Science.
Lithium, much like sodium, occurs in two forms: salt and metal. With sodium, salt (sodium chloride) is well known, while metal (Na) is a soft, gray material that reacts violently with water, producing sodium hydroxide (caustic soda or lye). Lithium metal behaves the same way, producing lithium hydroxide and hydrogen gas when placed in water (shown in the video). The ion, Li+, is present in lithium-ion batteries as well as in lithium salts, which are found in the ocean along with sodium and other salts. While pure lithium metal is unstable, it has the potential to revolutionize energy storage.
“The discovery is remarkable – it’s energetically quite difficult to convert lithium ion to lithium metal,” says Jeffrey Dick, Richard B. Wetherill Professor of the Department of Chemistry. “To think that microdroplets, found worldwide, can harbor such unexpected and unprecedented chemical reactivity is likely one of the greatest chemical findings of the century, in my opinion. Even though the Cooks’ group discovered many exciting chemistries in microdroplets, the latest discovery takes the cake. The implications span broad fields of study, now with potential impact on the field of energy storage and conversion.”
Cooks’ team created a simple method of converting lithium ions to lithium metal nanoparticles using an aqueous spray of lithium salt. Cooks disclosed the innovation to the Purdue Innovates Office of Technology Commercialization, which has applied for a patent to protect the intellectual property.
“Lithium metal, in the form of nanoparticles, is generated when charged microdroplets from a solution of lithium salt in water is sprayed onto a surface using nitrogen gas to drive the spray,” he explains. “This is surprising because lithium metal reacts immediately and violently with water in a reaction that releases a lot of energy (ca. 300 kJ/mol or 300 calories in common parlance). And this is important because the role of lithium in power storage, like batteries, depends on this reduction reaction. The fascinating chemistry at the surface of microdroplets drives this reaction, acting as a power source. In our experiment, nitrogen is reduced to ammonia while Li metals are oxidized. Ammonia, of course, as anyone who has driven through the Midwest countryside knows, is a major component of fertilizer.”
The team explored the idea of looking for lithium nanoparticles due to the persistence of lead author Dylan Holden and co-author Myles Edwards. At the time, Dr. Holden was an analytical chemistry PhD student working with Prof. Cooks. After demonstrating the ammonia production, Dr. Holden felt there was more to learn and took another 18 months to find and fully characterize the lithium metal nanoparticles. He and the team used a variety of surface analysis techniques at Purdue’s Birck Nanotechnology Center that distinguish Li+ from Lio.
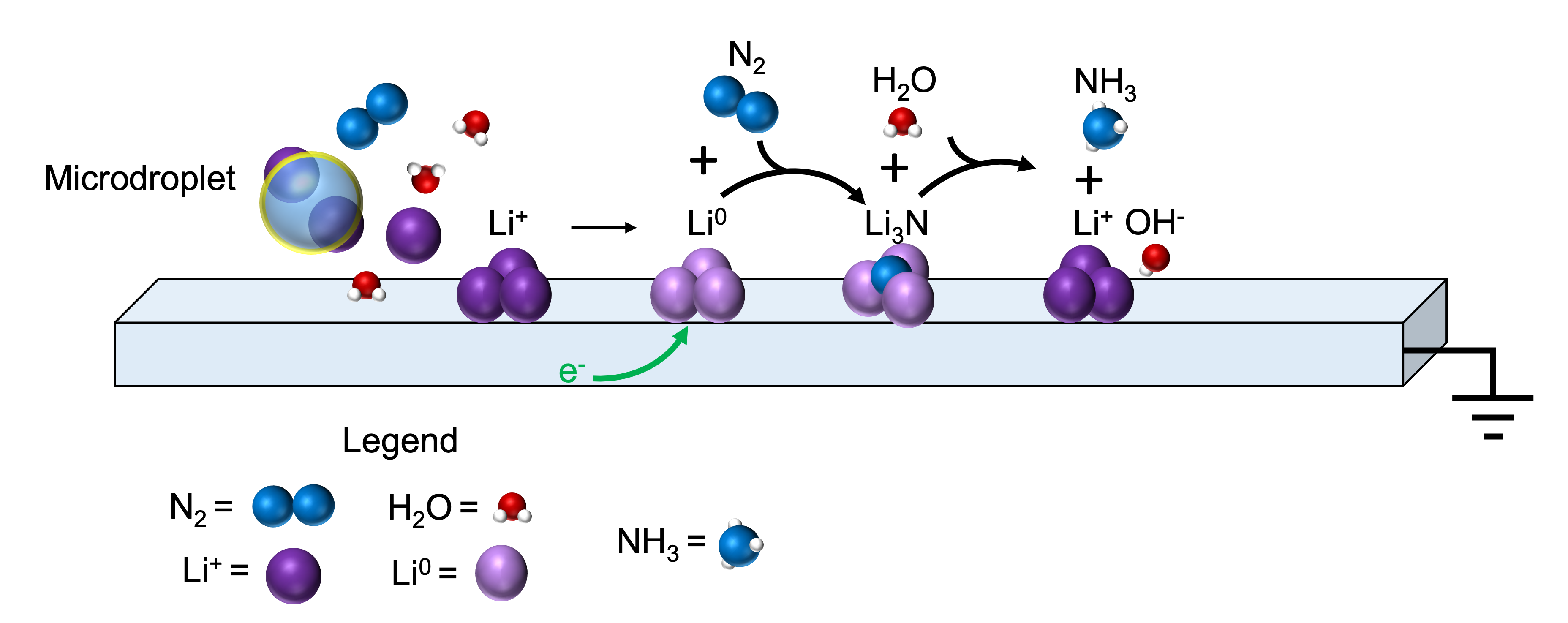
Other members of the research team are Myles Quinn Edwards, Ph. D. student in Analytical Chemistry, and Zhongxia Shang, Research Scientist at the Birck Nanotechnology Center. Cooks directed the project with the original aim of using microdroplet chemistry to fix nitrogen (N2) in the form of ammonia (NH3).
“I was as surprised as anyone at the formation of Li nanoparticles; even we sprayed metal salt solutions to give metal nanoparticles more than a decade ago,” said Cooks. “The surprise was that lithium metal formed in the presence of water. Dylan Holden led the experiments and his knowledge of the literature of nitrogen fixation, together with my teams’ unwillingness to stop the experiments after the first successful data had been taken, led directly to the observation of the lithium nanoparticles.”
Co-author Myles Edwards worked closely with Holden on the experiments and their interpretation while Shang helped perform the specialized surface science experiments needed to characterize the lithium nanoparticles.
“My lab builds and uses mass spectrometers (MS), widely used scientific instruments but little known to the public,” said Cooks. “The atomic bomb Manhattan Project depended on MS, so does the quality of your drinking water, the development of new drugs, hominid chronology, knowledge of atmospheric pollution, quality control in many industries, and much more. A very particular interest is the study of chemical reactions in microdroplets, which, in work published in 2011 and 2012, we found to be faster by up to a million times than conventional reactions. We are studying and using this phenomenon, trying to understand it in detail, and using it to make new compounds by chemical synthesis, including new drugs.”
Cooks is a member of the Purdue Institute for Cancer Research, Drug Discovery, Bindley Bioscience Center, and Purdue Institute of Inflammation, Immunology, and Infectious Disease.
“Purdue has had outstanding strength in analytical chemistry for decades - #1 ranking among US graduate programs,” says Cooks. “We have particular strength in mass spectrometry and chemical instrumentation. Analytical chemistry represents key infrastructure in the chemical sciences with implications across many disciplines besides chemistry.”
Cooks disclosed the innovation to the Purdue Innovates Office of Technology Commercialization, which has applied for a patent to protect the intellectual property. Industry partners interested in developing or commercializing the work should contact Dipak Narula, lead technology development liaison and assistant director of business development and licensing-physical sciences, at dnarula@prf.org, about track code 71250.
The work was funded by the Multi-University Research Initiative (MURI) of the Air Force Office of Scientific Research. In addition, Dylan Holden was supported by an NSF Graduate Research Fellowship.
About Purdue Chemistry
The James Tarpo Jr. and Margaret Tarpo Department of Chemistry is internationally acclaimed for its excellence in chemical education and innovation, boasting two Nobel laureates in organic chemistry, the #1 ranked analytical chemistry program, and a highly successful drug discovery initiative that has generated hundreds of millions of dollars in royalties. chem.purdue.edu.
Contributors:
Graham Cooks, Henry Bohn Hass Distinguished Professor, Department of Chemistry
Dylan T. Holden, Ph.D. in Analytical Chemistry, currently a postdoctoral fellow at Harvard University
Myles Quinn Edwards, Ph. D. student in Analytical Chemistry
Jeffrey Dick, Richard B. Wetherill Professor of the Department of Chemistry
Zhongxia Shang, Ph.D. Technical Staff (Research Analyst Lead) Brick Nanotechnology Center
Graphic provided by Myles Edwards and Dylan Holden.
Written by Cheryl Pierce, Purdue University College of Science