Lyudmila Slipchenko

- Professor | Physical/Theoretical Chemistry
- Email: lslipchenko@purdue.edu
- Phone: 45255
- Office: 265H WTHR
- Professor Lyudmila Slipchenko's individual homepage
The goal of our research program is to understand the fundamental laws that control chemistry in the condensed phase, using quantum chemistry tools. The environment can affect chemical processes in different ways. For example, a solvent may completely change the character of the electronic states of a solute and create new, so called charge transfer-to-solvent states. This occurs, for example, when iodide anion is solvated in water. On the other hand, the protein environment does not create new electronic states in the retinal chromophore in visual rhodopsin, but modifies the potential energy surfaces of the chromophore states and the coupling between them. Thus, it is often unclear if we can think about molecular electronic states as just being perturbed by the solvent, or if we need to completely depart from the gas phase picture even for qualitative understanding. Additional complexity arises when dynamics comes into play, since solvent relaxation may occur in different time scales and by different pathways, often resulting in controversial spectroscopic signatures. While these are fundamental phenomena, their understanding can lead to practical ways of controlling chemistry in the condensed phase.
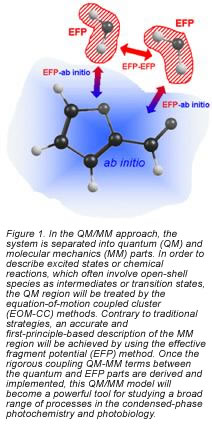
Theoretical modeling of a chemical process in solution or on a surface is challenging due to the dramatic increase in the number of degrees of freedom of the combined system, exacerbated by the complexity and diversity of the underlying mechanisms and pathways. This makes computer simulations very demanding. In our group, we develop robust computational tools that will facilitate accurate and revealing investigations of chemical and biological processes in an environment. In particular, we plan to combine state-of-the-art ab initio excited state methods in the equation-of-motion coupled-cluster (EOM-CC) family and the sophisticated model potential called the effective fragment potential (EFP) method in a novel and efficient hybrid QM/MM (quantum mechanics/molecular mechanics) method (see Fig. 1).
By using this new technique, we will be able to investigate a broad range of intriguing chemical and biological processes in which solvent effects are crucial and which are also of practical importance. For example, in the photocycle of the photoactive yellow protein (PYP) we will examine how the protein environment directs and constrains the photoisomerization in the PYP chromophore. Another class of problems for which solvent effects are crucial is related to environmental pollution. We will investigate the possibility of industrial usage of zeolite cages for selective hydrocarbon oxidation. This would significantly decrease the environmental burden by eliminating the waste and high-energy losses, which currently accompany industrial production of partially oxidized hydrocarbons. We are also fascinated by recent developments in the field of molecular electronics, and would like to learn how the electronic structure and conductivity in organic conjugated polymers is related to their morphology.
Education
- B.S., Moscow Institue of Physics and Technology, 1998
- M.S., Moscow Institue of Physics and Technology, 2000
- Ph.D., Univeristy of Southern California, 2005